Camptothecin prodrug monomer and polymeric prodrug amphipathic molecules thereof as well as preparation method and application of camptothecin prodrug monomer and polymeric prodrug amphipathic molecules
A technology of amphiphilic molecules and camptothecin, which is applied in the field of medicinal chemistry and biodegradable medical polymers, can solve the problems of lack of tumor microenvironment response release characteristics and limited drug loading, and achieve improved water solubility and stable stability. The effect of controlled release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1: Single-ended modification of hydroxyethyl disulfide
[0079] As shown in Scheme 1, in a 500mL round bottom flask, weigh hydroxyethyl disulfide (6.16g, 40mmol), triethylamine (6.07g, 60mmol) and 200mL of dry THF, cool in an ice-water bath and stir with a magnet Under certain conditions, methacryloyl chloride (4.18 g, 40 mmol, in 100 mL THF) was slowly added dropwise, and after the dropwise addition was completed, it was stirred overnight at room temperature. The triethylamine hydrochloride by-product generated by the reaction was removed by filtration, and the filtrate was collected to remove the solvent by rotary evaporation, then dissolved in ethyl acetate, washed with water and saturated NaCl aqueous solution three times, and the organic phase was collected and dried with anhydrous magnesium sulfate, then filtered, concentrate. The crude product was separated and purified by silica gel column chromatography, and eluted with ethyl acetate:petroleum ether=1:...
Embodiment 2
[0080] Example 2: Preparation of Reductively Responsive Camptothecin Prodrug Monomer CPTM
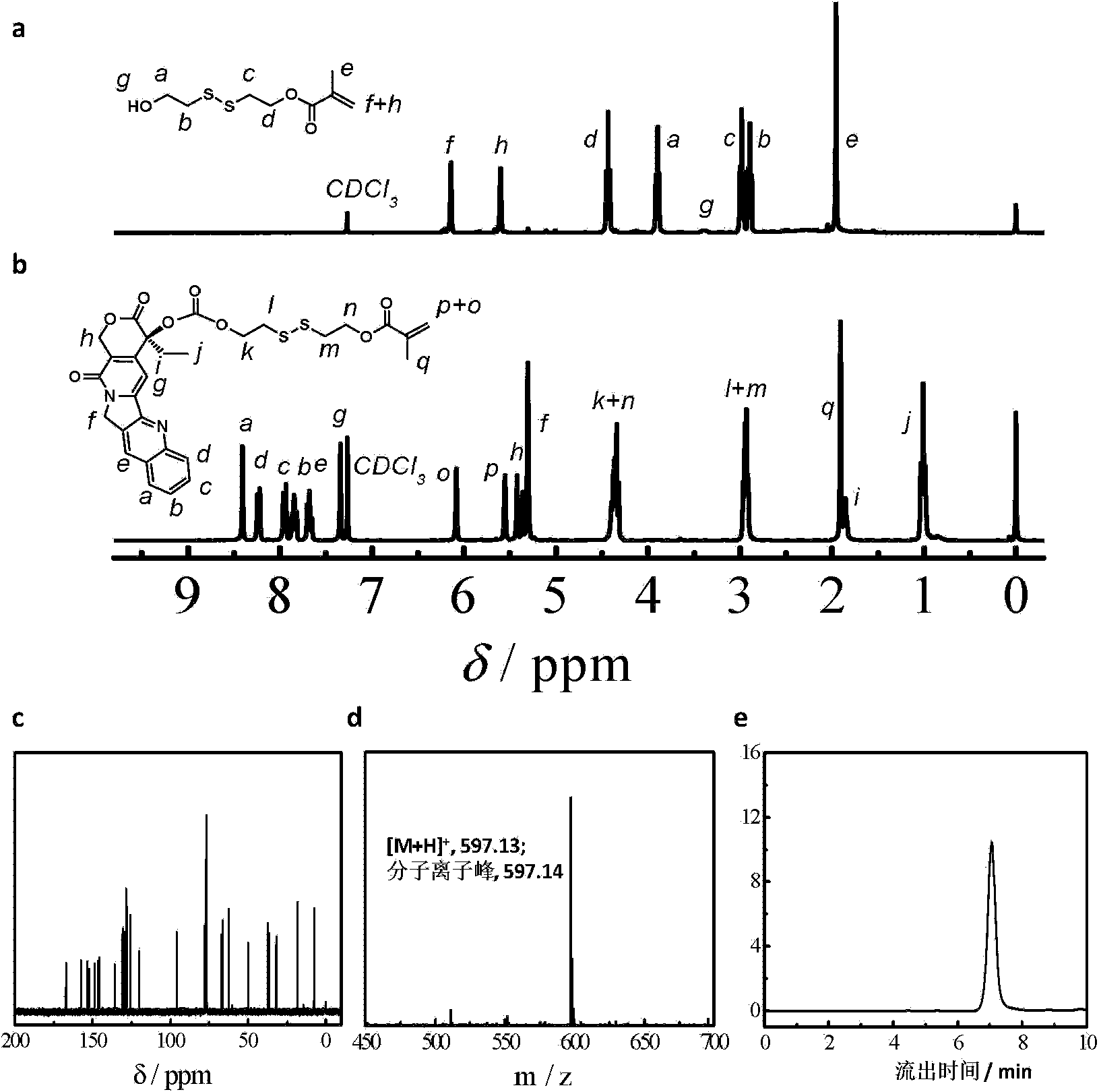
[0081]According to the above scheme 1, dissolve and disperse CPT (2.0g, 5.74mmol) and DMAP (2.11g, 17.3mmol) in 50mL of dry dichloromethane, add triphosgene (0.567g, 1.92mmol), react at room temperature for 30min, and then add dropwise A solution of the above product (Formula 2) in dry THF (1.40 g, 6.31 mmol in 15 mL THF) was contained. A white precipitate formed after overnight reaction. After filtration, the filtrate was collected, concentrated, dissolved in ethyl acetate, washed with water and saturated NaCl aqueous solution three times, and the organic phase was collected and dried over anhydrous magnesium sulfate. The crude product was separated and purified by silica gel column chromatography, and eluted with ethyl acetate to obtain a light gray solid powder (2.92 g, yield: 86%). figure 1 (b) shows the hydrogen spectrum of the CPTM prepared in this example: 1 H NMR (CDCl 3 , δ...
Embodiment 3
[0082] Embodiment 3: Polyethylene glycol monomethyl ether end group RAFT modification
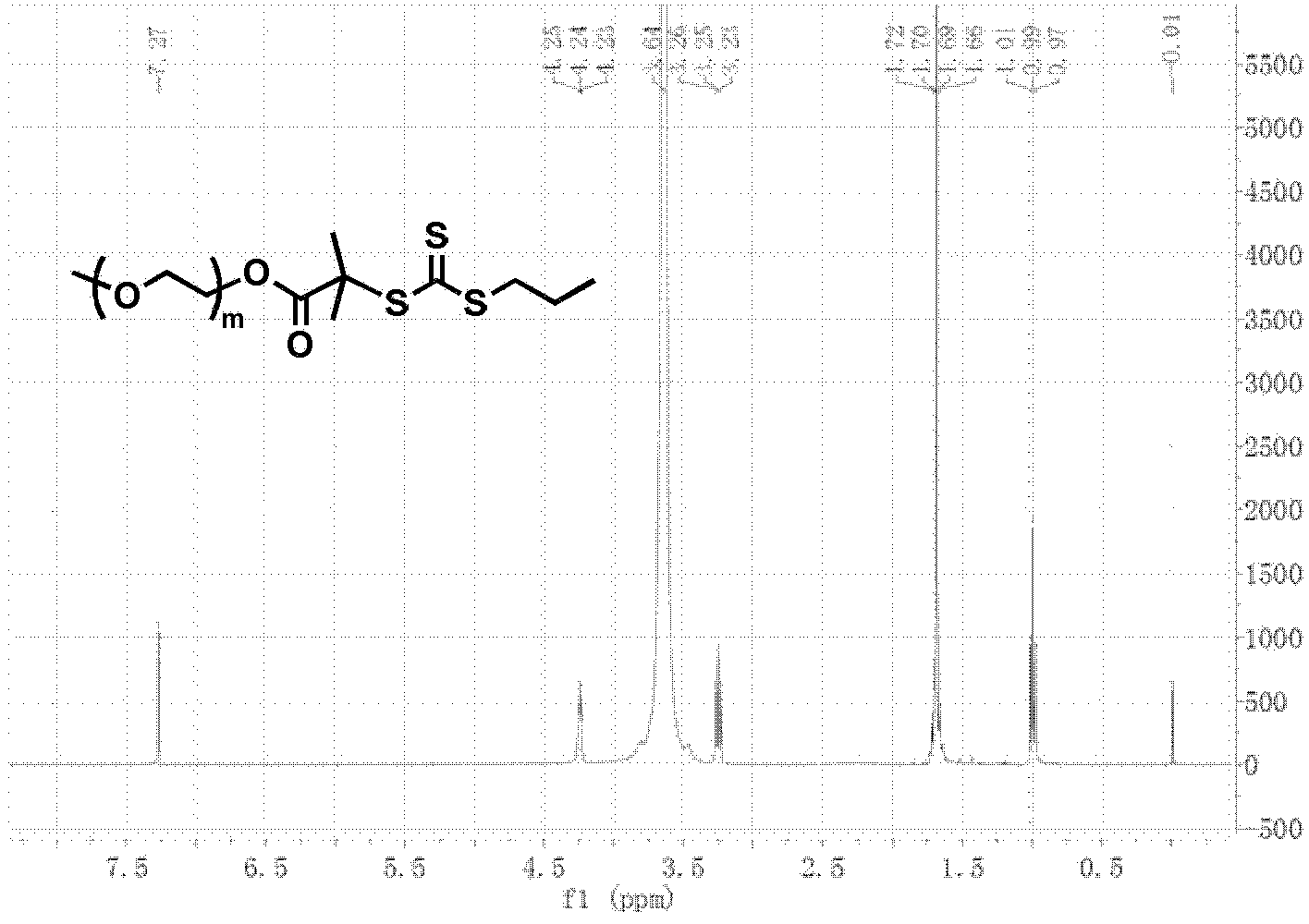
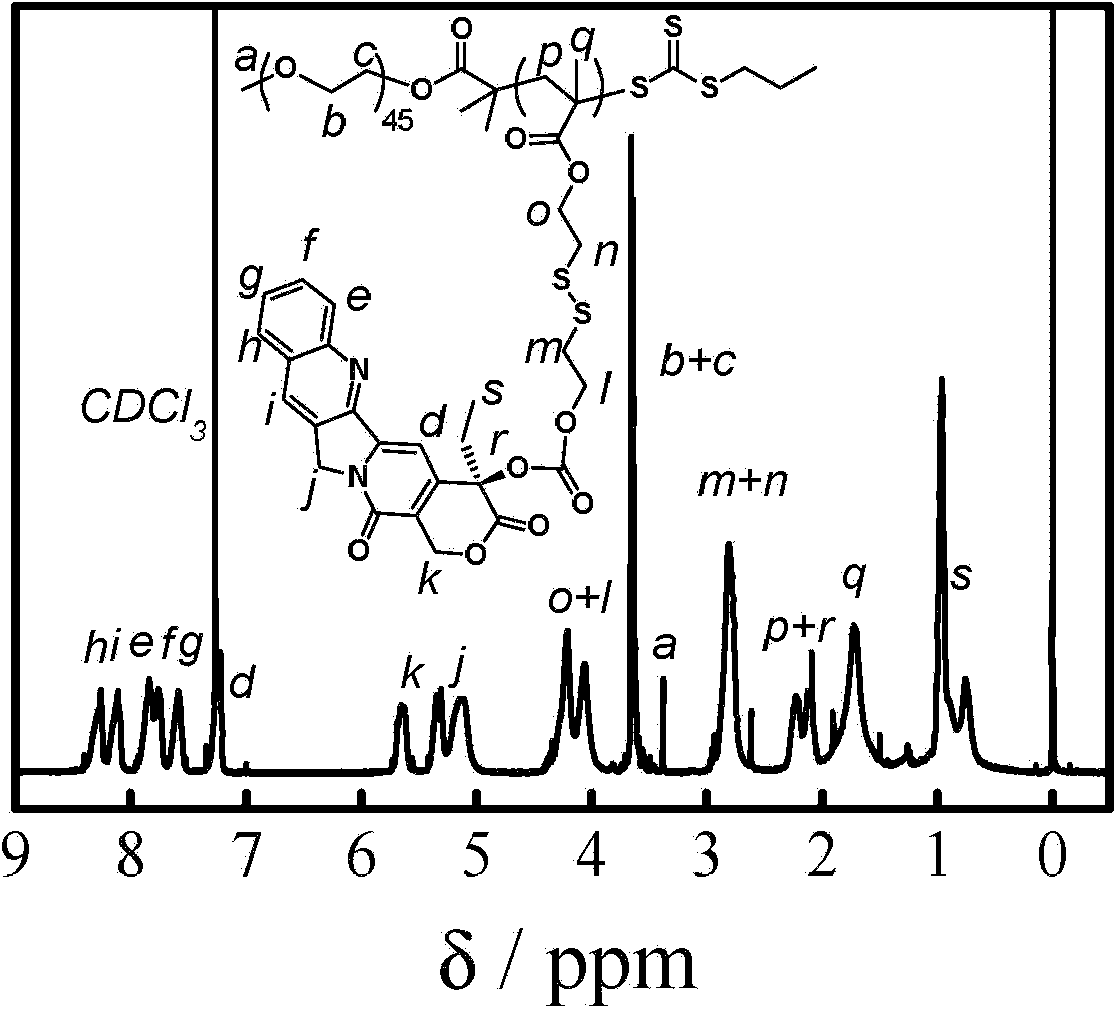
[0083] According to the above scheme 2, polyethylene glycol monomethyl ether (PEG 45 -OH, 4.0g, 2.0mmol) was dissolved in anhydrous toluene (25mL), and most of the solvent was removed by azeotroping under reduced pressure at 50°C, then the small molecule RAFT reagent (1.09g, 4.0mmol) of formula 3 was added and dried dichloromethane (100mL), cooled to 0°C in an ice-water bath, dicyclohexylcarbodiimide (0.83g, 4.0mmol) and N,N-dimethylaminopyridine (49mg, 0.4mmol) were added dropwise in dichloromethane mixture. Stir the reaction at room temperature for 48 hours, remove the generated by-product salt by filtration, concentrate under reduced pressure, precipitate in excess anhydrous ether, filter and dry, dissolve dichloromethane and then precipitate with anhydrous ether. The compound of formula 4 can be obtained by repeating three times. figure 2 shows the polyethylene glycol monomethyl eth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| electrical resistivity | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com