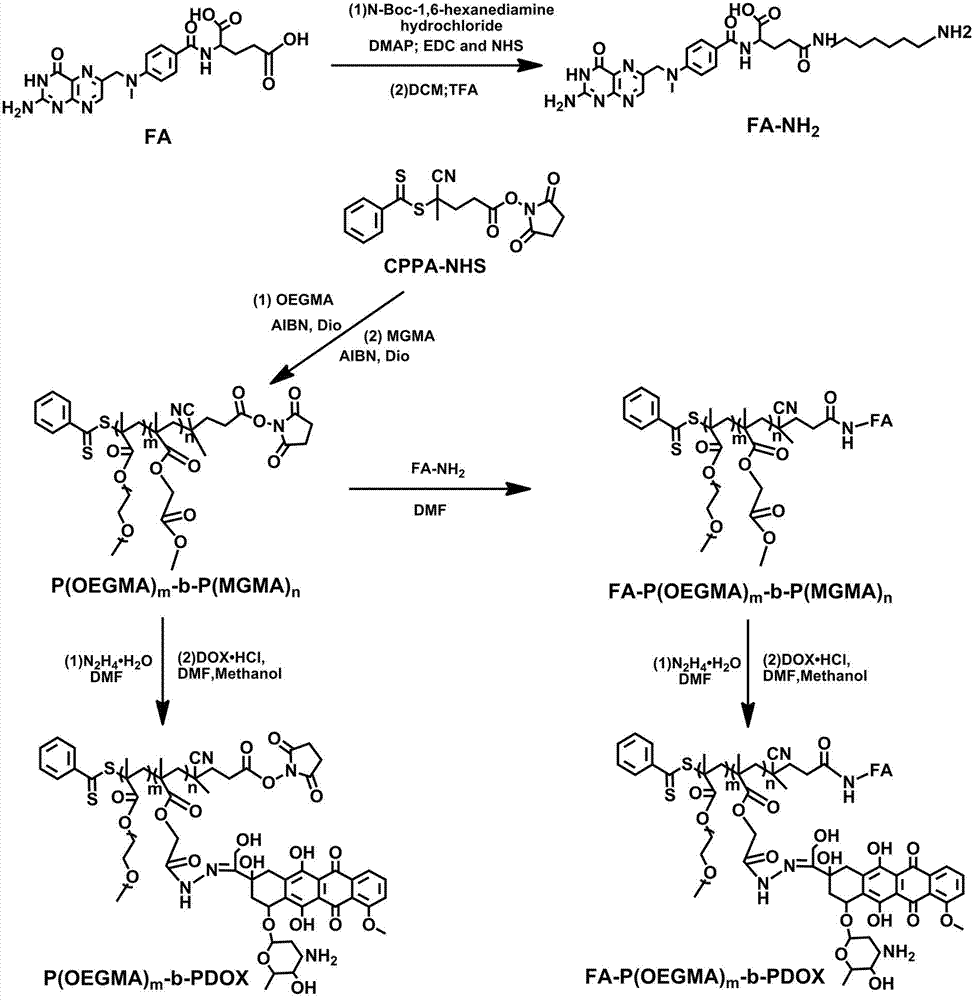
Preparation method of linear diblock polymeric prodrugs with pH simulative responsibility
A stimuli-responsive, di-block technology, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of damage to normal cells and tissues, poor water solubility of anti-tumor drugs, immune power loss etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Preparation of pH-stimulus-responsive polymeric prodrugs
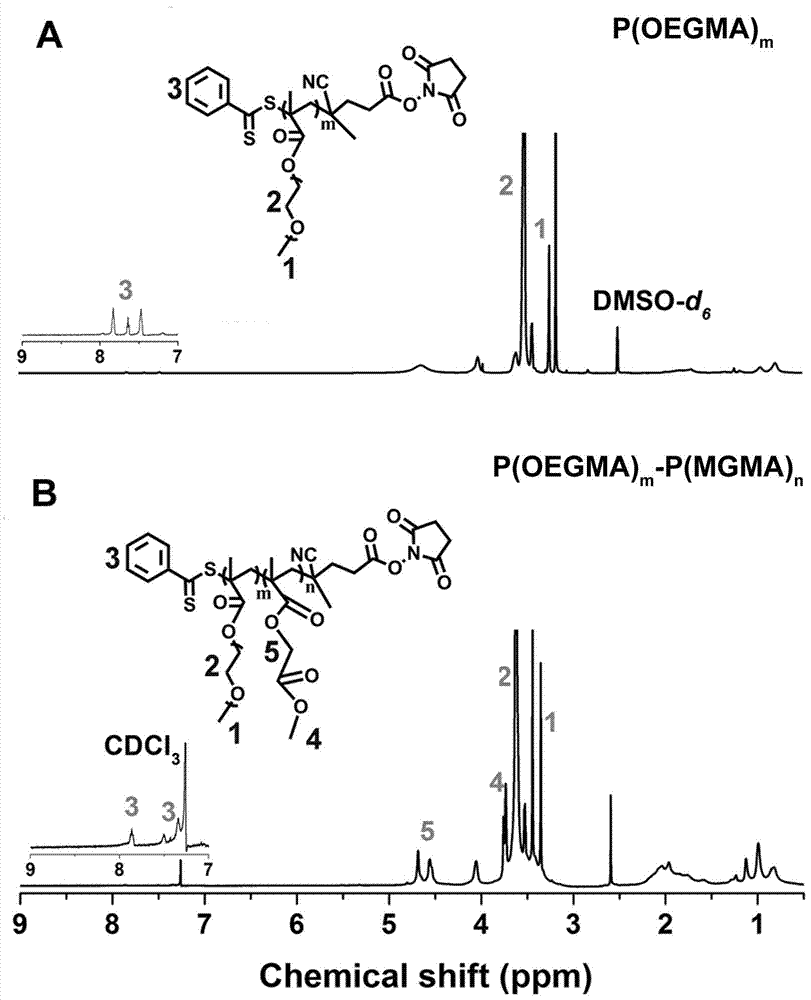
[0045] 1. Preparation of P(OEGMA) m : Under argon (Ar, 2-10Pa) conditions, CPPA-NHS is used as the macroinitiator of RAFT reaction, and it is dissolved in 1,4-dioxane together with ethylene glycol methacrylate (OEGMA) (Dio), after adding azoisobutyronitrile (AIBN), freeze-thaw cycle three times, react at 70°C in the dark for 24 hours, freeze with liquid nitrogen to terminate the reaction, thaw, add 3~5mL methanol to dilute, and use A dialysis bag with a molecular weight cut-off (MWCO) of 3500Da was dialyzed in methanol for 24 hours, concentrated and then deuterated-dimethyl sulfoxide (DMSO-d6) was used as a solvent (DMSO-d6) to measure the hydrogen spectrum. The results are as follows: figure 2 As shown in (A), 1 (3.24 ppm) and 2 (3.60 ppm) are the signal peaks of methoxy and methylene hydrogen on OEGMA, respectively, indicating that the hydrophilic monomer P(OEGMA) m successfully synthesized;
[...
Embodiment 2
[0051] Example 2 Preparation of a pH-stimulus-responsive polymeric prodrug, which also has targeting
[0052] (I) Preparation of FA-NH2: Dissolve folic acid (FA) in anhydrous DMF in an ice bath (temperature ≤0°C) and argon (Ar, 2-10Pa), add catalysts EDC and NHS, After stirring for 0.5h, add NH2-BOC dissolved in TEA-containing DMF to form a mixed solution, react at room temperature (25°C) for 24h, slowly add dropwise to the secondary water under constant stirring, filter, and obtain FA-NH after vacuum drying -BOC 。 Add DCM and TFA solvent and stir for 0.5h, concentrate, add DCM and TEA, stir at room temperature (25°C) in the dark for 24h, rotate at 5000r / min, centrifuge for 3min, wash twice with DCM, and vacuum dry to obtain FA-NH 2 ;
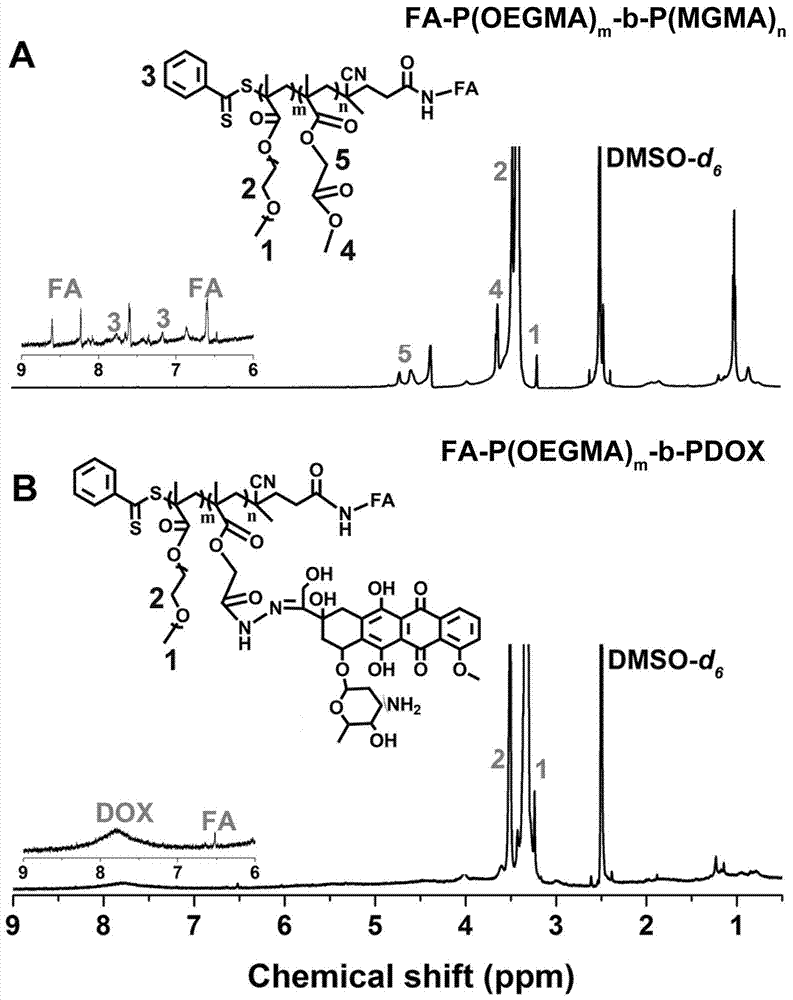
[0053](II) Preparation of FA-P (OEGMA) m -b-P(MGMA) n : P(OEGMA) at room temperature (25°C) with argon (Ar, 2-10Pa) m -b-P(MGMA) n with FA-NH 2 Dissolved in TEA-containing DMSO solution, reacted in the dark for 48 hours, precipitated in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com