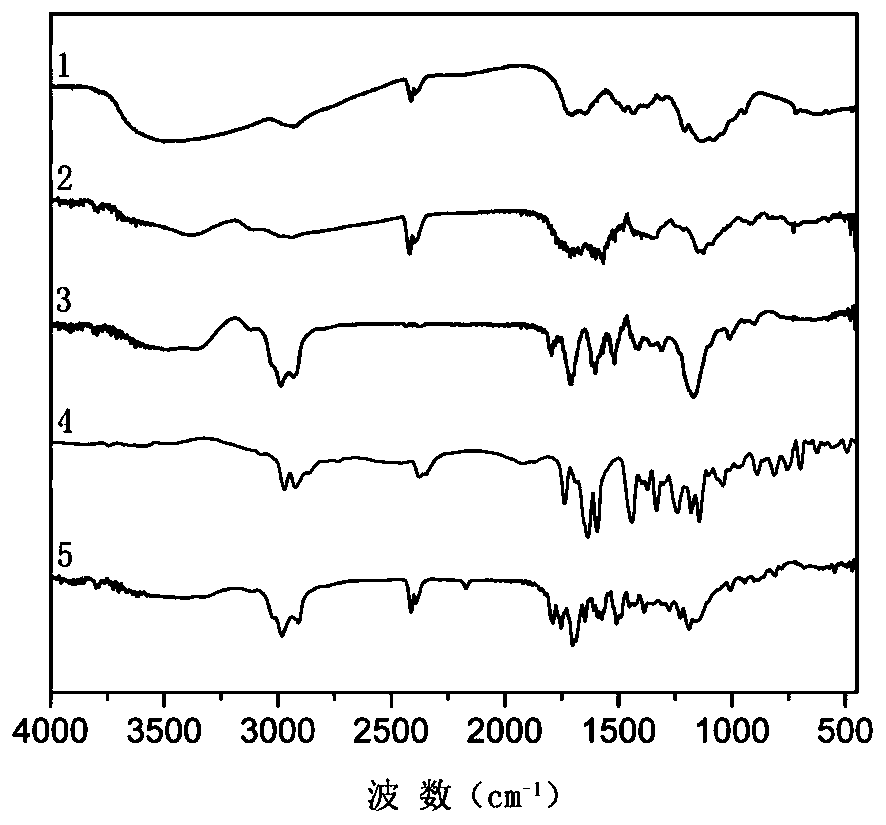
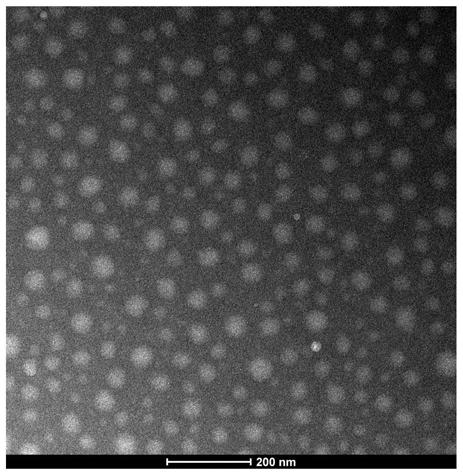
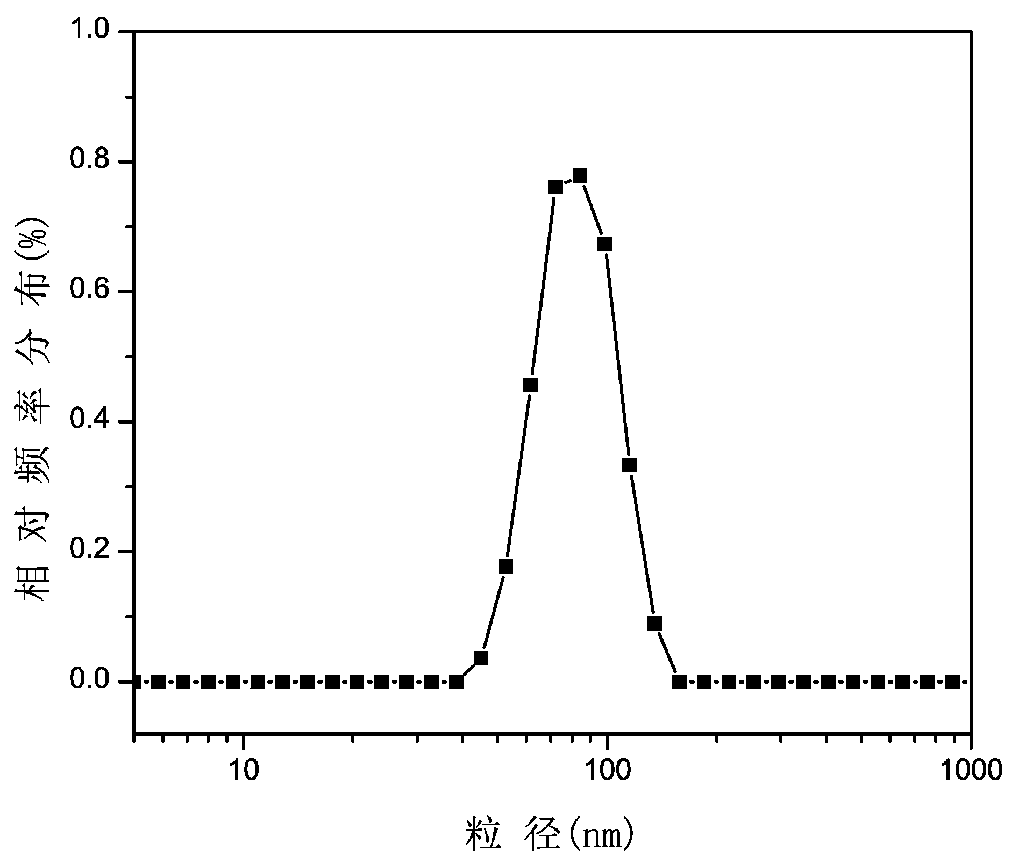
Chitosan-based multi-environment-responsive type polymeric prodrug micelle and preparation method thereof
A technology of chitosan and polymer, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problem of low solubility of gambogic acid, limited clinical application, easy leakage of drugs, etc. problem, to achieve the effect of inhibiting growth and reproduction, improving therapeutic effect and improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Preparation of folic acid-chitosan-poly(N-isopropylacrylamide) polymer: Folic acid-chitosan conjugate and amino-terminated poly(N-isopropylacrylamide) 1:4 Add the mixture of the weight ratio to 0.3% (v / v) acetic acid solution, add 1% (v / v) polysorbate 80, stir at room temperature for 1h, slowly drop glutaraldehyde solution into the reaction system, wherein folic acid -The mass ratio of chitosan conjugate, amino-terminated poly(N-isopropylacrylamide), and glutaraldehyde is 2:8:1. The reaction was continued for 1 h, and folic acid-chitosan-poly(N-isopropylacrylamide) polymer was obtained after freeze-drying.
[0039] (2) Preparation of gambogic acid and folic acid-chitosan-poly(N-isopropylacrylamide) graft polymer: gambogic acid, 1,3-dicyclohexylcarbodiimide and 4-dimethyl Aminopyridine was added to 10 mL of N,N-dimethylformamide to dissolve completely, and stirred at room temperature for 1 h in the dark. Dissolve the folic acid-chitosan-poly(N-isopropylacrylamide) ...
Embodiment 2
[0051] (1) Preparation of folic acid-chitosan-poly(N-isopropylacrylamide) polymer: the mixture of folic acid-chitosan conjugate and amino-terminated poly(N-isopropylacrylamide) Add 1:4 weight ratio to 0.3% (v / v) acetic acid solution, add 1% (v / v) poloxamer 188, stir at room temperature for 1 hour, then slowly drop glutaraldehyde solution into the reaction system, The mass ratio of folic acid-chitosan conjugate, amino-terminated poly(N-isopropylacrylamide), and glutaraldehyde is 2:8:1. Stirring is continued, and the folic acid-chitosan-poly(N-isopropylacrylamide) polymer is obtained after freeze-drying.
[0052] (2) Graft polymer of gambogic acid and folic acid-chitosan-poly(N-isopropylacrylamide): gambogic acid, 1,3-dicyclohexylcarbodiimide and 4-dimethylamino Pyridine was added to 10 mL of N,N-dimethylformamide to dissolve completely, and stirred at room temperature for 1 h in the dark. Dissolve the folic acid-chitosan-poly(N-isopropylacrylamide) polymer prepared in step (1...
Embodiment 3
[0055] (1) Preparation of folic acid-chitosan-poly(N-isopropylacrylamide) polymer: the mixture of folic acid-chitosan conjugate and amino-terminated poly(N-isopropylacrylamide) Add 1:4 weight ratio to 0.3% (v / v) acetic acid solution, add 1% (v / v) sodium dodecyl sulfate, stir at room temperature for 1 hour, then slowly drop glutaraldehyde solution into the reaction system , wherein the mass ratio of folic acid-chitosan conjugate, amino-terminated poly(N-isopropylacrylamide), and glutaraldehyde is 2:8:2. Stirring is continued, and the folic acid-chitosan-poly(N-isopropylacrylamide) polymer is obtained after freeze-drying.
[0056] (2) Graft polymer of gambogic acid and folic acid-chitosan-poly(N-isopropylacrylamide): gambogic acid, 1,3-dicyclohexylcarbodiimide and 4-dimethylamino Pyridine was added to 10 mL of N,N-dimethylformamide to dissolve completely, and stirred at room temperature for 1 h in the dark. Dissolve the folic acid-chitosan-poly(N-isopropylacrylamide) polymer p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com