A redox-responsive hyperbranched polyprodrug nanomicelle and its preparation method and application
A hyperbranched polymer and nanomicelle technology, which is applied in the field of biomedical materials, can solve problems such as increased blood circulation time, and achieve the effects of low cytotoxicity, good stability, and long blood circulation time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: the synthesis of curcumin-SS-methacrylate
[0061] Take 444 mg of compound 1 (2 mmol) and 0.7 mL of N,N-diisopropylethylamine (DIPEA, 4 mmol) and dissolve in 50 mL of anhydrous tetrahydrofuran (THF). Dissolve 297 mg of triphosgene in 5 mL of anhydrous THF and add it dropwise to the above solution in an ice bath. After reacting for 3 hours, remove the insoluble matter by suction filtration. Take 736 mg of curcumin (2 mmol) and 0.7 mL of DIPEA and dissolve it in 10 mL of anhydrous THF, add the above-mentioned suction-filtered solution to the solution dropwise in an ice bath, and react overnight. After the reaction, first spin dry THF, add ethyl acetate to dissolve, wash with 0.1M dilute hydrochloric acid two to three times, then wash with water two to three times, take the upper organic phase, add anhydrous magnesium sulfate to remove water , filtered, and then the ethyl acetate was spin-dried to obtain a crude product. The crude product was passed through...
Embodiment 2
[0066] Example 2: Synthesis of Poly(ACPP-SS-CUR)
[0067] Take 259mg curcumin-SS-methacrylate (0.42 mmol), 19.64mg ACPP (2-((2-(acryloyloxy)-ethyl)-disulfanyl)ethyl 4-cyano-4 -(((Propylthiocarbonylthiocarbonyl)-thio)pentanoate)) (0.042mmol) and 1.37mg AIBN (azobisisobutyronitrile, 0.0084 mmol) to a round bottom flask, add 1mL DMSO solution Dissolve, then freeze and pump in liquid nitrogen for 30 minutes, thaw again, pass argon gas for 2-3 minutes, and then freeze and pump in liquid nitrogen for 30 minutes; repeat the above freezing and pumping process for 3 times; react the solution in an oil bath at 90°C After 30h, cool. The above solution was added dropwise to ether to precipitate, centrifuged, and the precipitate was collected. Add 2-3 mL of tetrahydrofuran to make it dissolve, and then make it precipitate in ether, repeat the precipitation three times, and take the precipitate. The purified precipitate was vacuum-dried for 24 h to obtain a redox-responsive hyperbranched...
Embodiment 3
[0070] Example 3: Synthesis of Poly(ACPP-SS-CUR-OEGMA)
[0071] 50 mg of ACPP-SS-CUR prepared in Example 2, 250 mg of OEGMA and 1.95 mg of AIBN were dissolved in 1.5 mL of DMSO. Then freeze and pump in liquid nitrogen for 30 minutes, thaw again, pass argon gas (2-3 minutes), and freeze and pump in liquid nitrogen for 30 minutes, repeat the above freezing and pumping process for a total of 3 times; After reacting for 30h, it was cooled. The above solution was added dropwise to ether to precipitate, centrifuged, and the precipitate was collected. Add a small amount of dichloromethane to dissolve it, and then make it precipitate in ether, repeat the precipitation three times, and take the precipitate. The purified precipitate was vacuum-dried for 24 h to obtain a redox-responsive hyperbranched polyprodrug Poly (ACPP-SS-CUR-OEGMA).
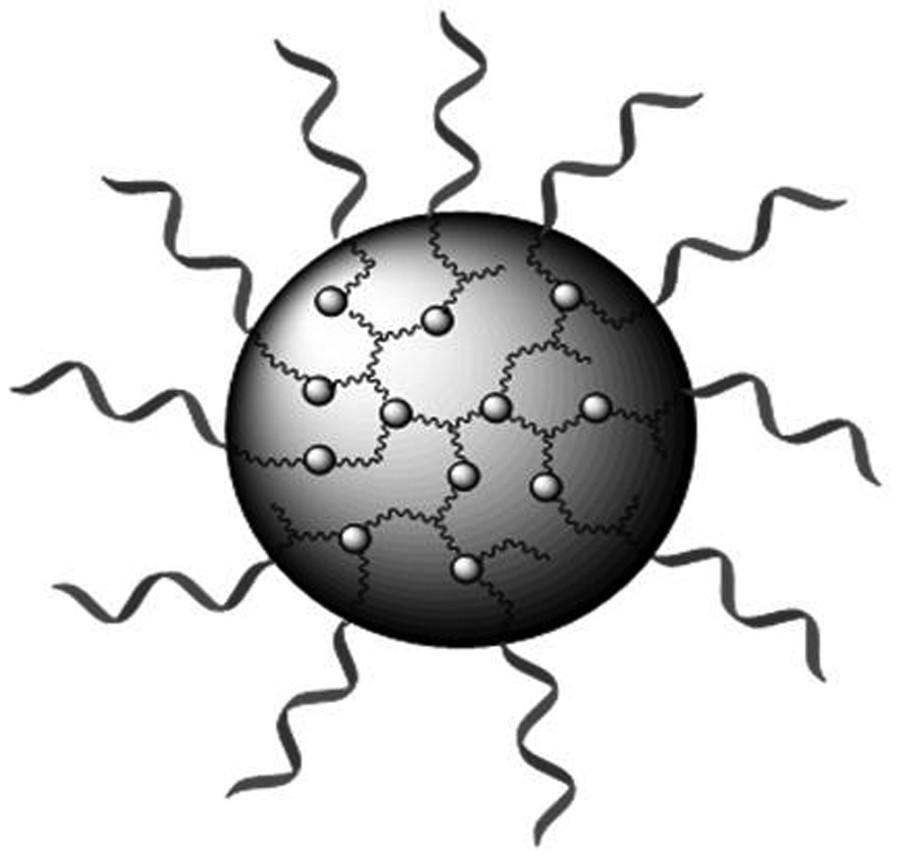
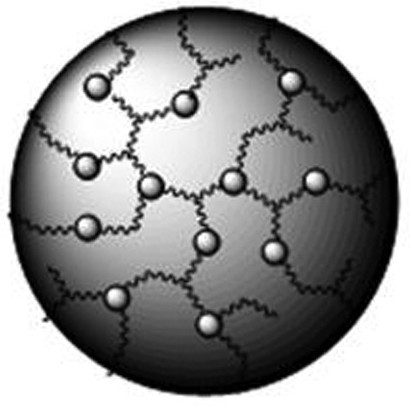
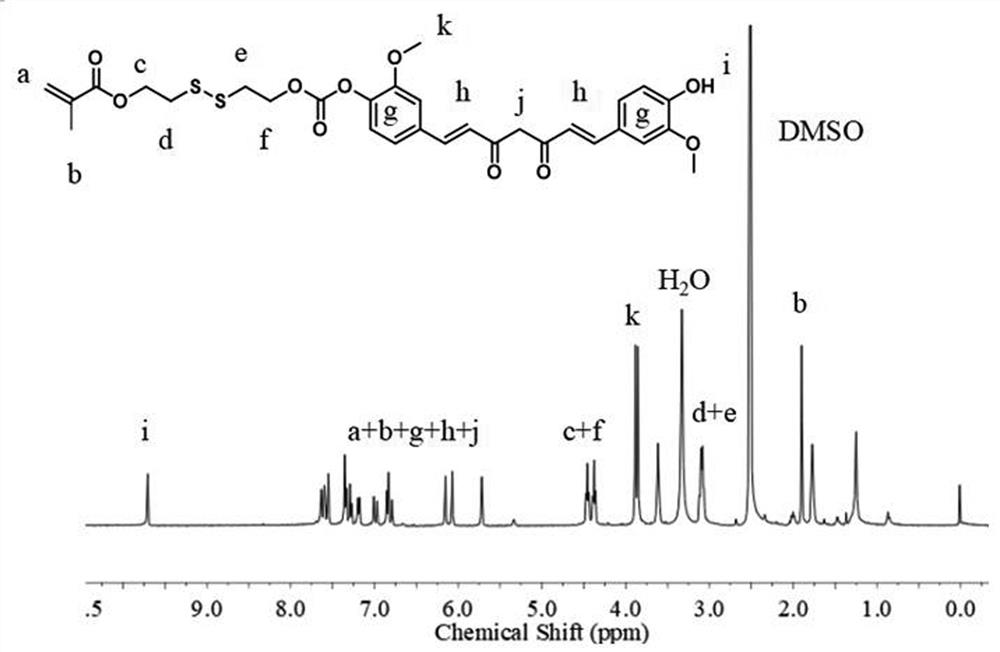
[0072] Schematic diagram of the redox-responsive hyperbranched polyprodrug Poly (ACPP-SS-CUR-OEGMA) is shown in Figure 5 ; H NMR spectrum, see ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com