Methods of treating her2 positive cancer with her2 receptor antagonist in combination with multi-arm polymeric conjugates of 7-ethyl-10-hydroxycamptothecin
A technology of receptor antagonist and camptothecin, which is applied in the field of treating HER2-positive cancers in mammals, can solve problems such as the danger of chemotherapy, and achieve the effects of improving therapeutic effect, enhancing side effects, and inhibiting tumor growth and/or proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0297] Alternative embodiments for the treatments described herein include:
[0298]
[0299] wherein (n) is an integer from about 28 to 341 such that the total molecular weight of the polymeric portion of the compound of formula (II) is from about 5,000 to about 60,000 Daltons, preferably about 20,000 or 40,000 Daltons.
[0300] In other embodiments, the treatments described herein utilize the polymeric compounds described in WO2005 / 028539, the contents of which are incorporated herein by reference in their entirety.
[0301] C. HER2 antagonists
[0302] Many types of cancer are associated with elevated ER2 protein and gene levels. HER2 proteins catalyze the transfer of terminal phosphates from ATP to tyrosine residues of protein substrates. HER2 receptor antagonists and HER2 antagonists generally refer to compounds that inhibit the function or expression of the HER2 protein or gene. HER2 receptor antagonists can inhibit HER2 receptor function directly or through downst...
Embodiment 1
[0382] Example 1. Toxicity Data
[0383] The maximum tolerated dose ("MTD") of 4Arm-PEG-Gly-(7-ethyl-10-hydroxycamptothecin) (compound 9) was studied using nude mice. Mice were monitored for 14 days for mortality and signs of disease and sacrificed when body weight loss > > 20% of pre-treatment body weight.
[0384] Table 2 below shows the maximum tolerated dose for each compound (single and multiple dose administration). Mice were administered each dose of the multi-dose administration on alternate days for 10 days and then observed for an additional 4 days, thus a total of 14 days.
[0385] Table 2. MTD data in nude mice
[0386]
[0387] The MTD of 4Arm-PEG-Gly-(7-ethyl-10-hydroxycamptothecin) (compound 9) was found to be 30 mg / kg when administered as a single dose and 10 mg when administered in multiple doses (q2d x 5) / kg.
Embodiment 2
[0388] Example 2. Properties of PEG conjugates
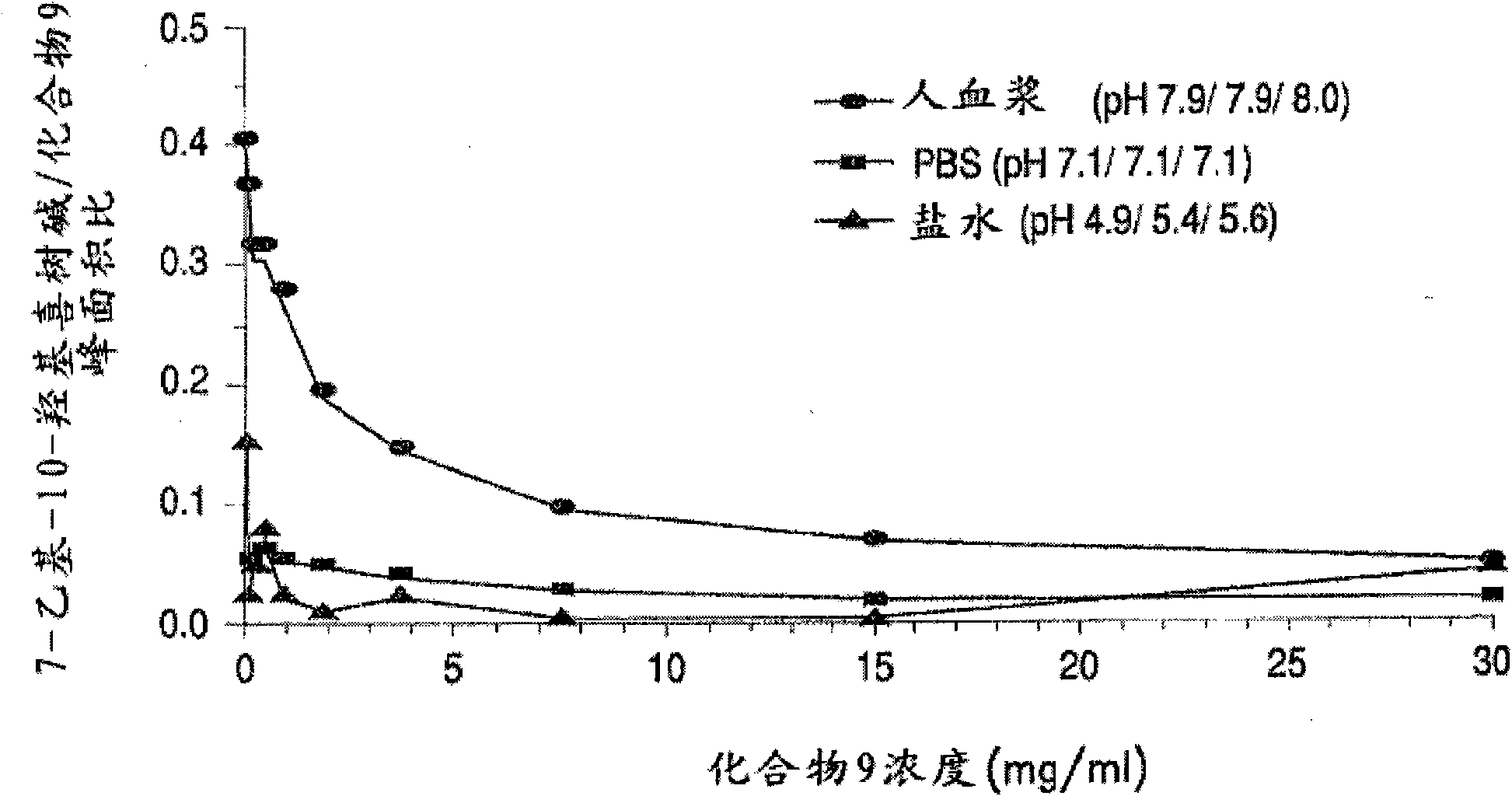
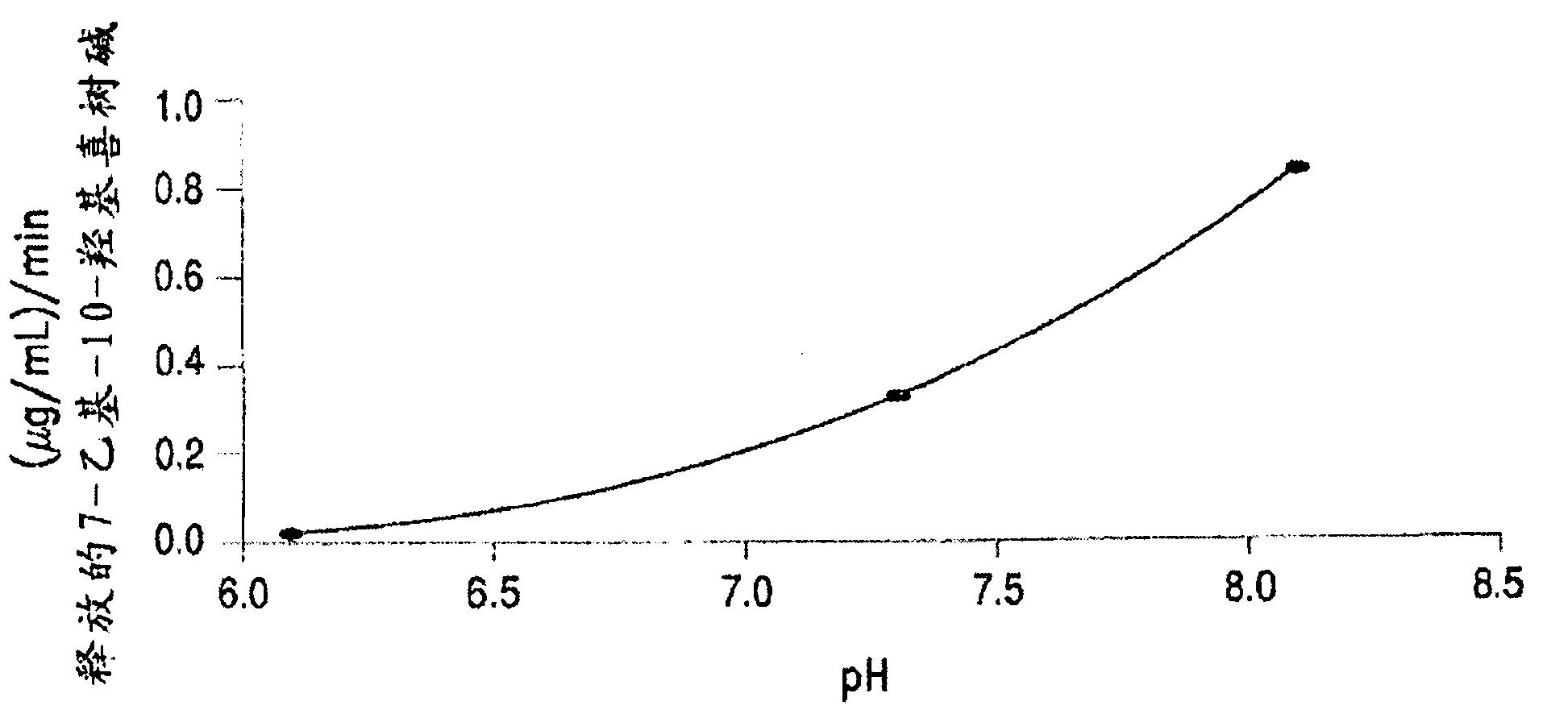
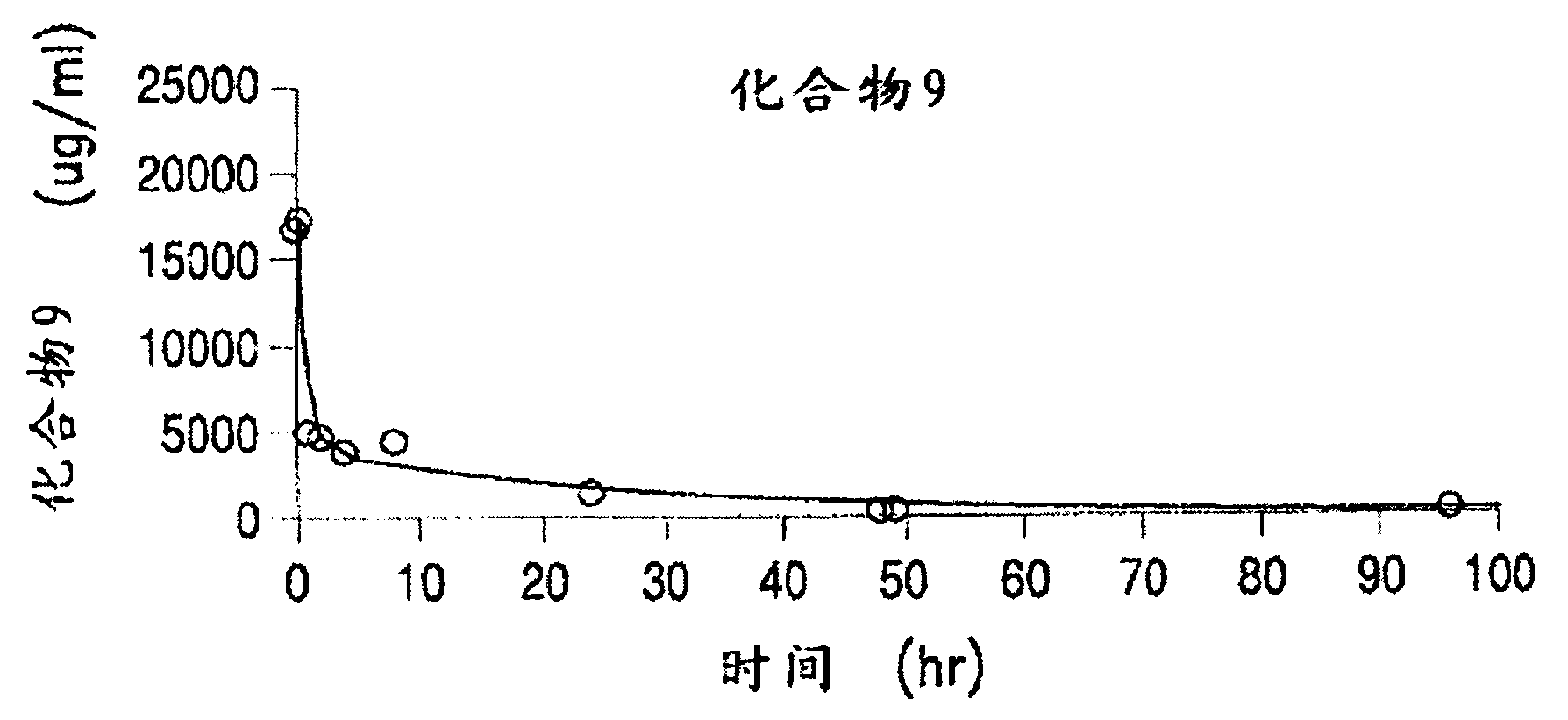
[0389] Table 3 below shows the solubility of 4 different PEG-(7-ethyl-10-hydroxycamptothecin) conjugates in saline solution. All four PEG-(7-ethyl-10-hydroxycamptothecin) conjugates showed good solubility up to 4 mg / mL equivalent to 7-ethyl-10-hydroxycamptothecin. In human plasma, 7-ethyl-10-hydroxycamptothecin was released stably from the PEG conjugate with a doubling time of 22 to 52 minutes, and the release exhibited pH and concentration as described in Example 3 below. rely.
[0390] Table 3. Properties of PEG-7-ethyl-10-hydroxycamptothecin conjugates
[0391]
[0392] a 7-Ethyl-10-hydroxycamptothecin is insoluble in saline
[0393] b PEG conjugate half-life
[0394] c Rate of formation of 7-ethyl-10-hydroxycamptothecin from conjugates
[0395] The PEG-Gly-7-ethyl-10-hydroxycamptothecin conjugate exhibits good stability in saline and other aqueous media for up to 24 hours at room temperature.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com