Preparation method of pH-responsive amphiphilic rod-like adriamycin polymer prodrug
An amphiphilic and doxorubicin technology, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of strong toxic and side effects, poor blood circulation stability, and low medical efficiency, etc. problems, to achieve effective treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
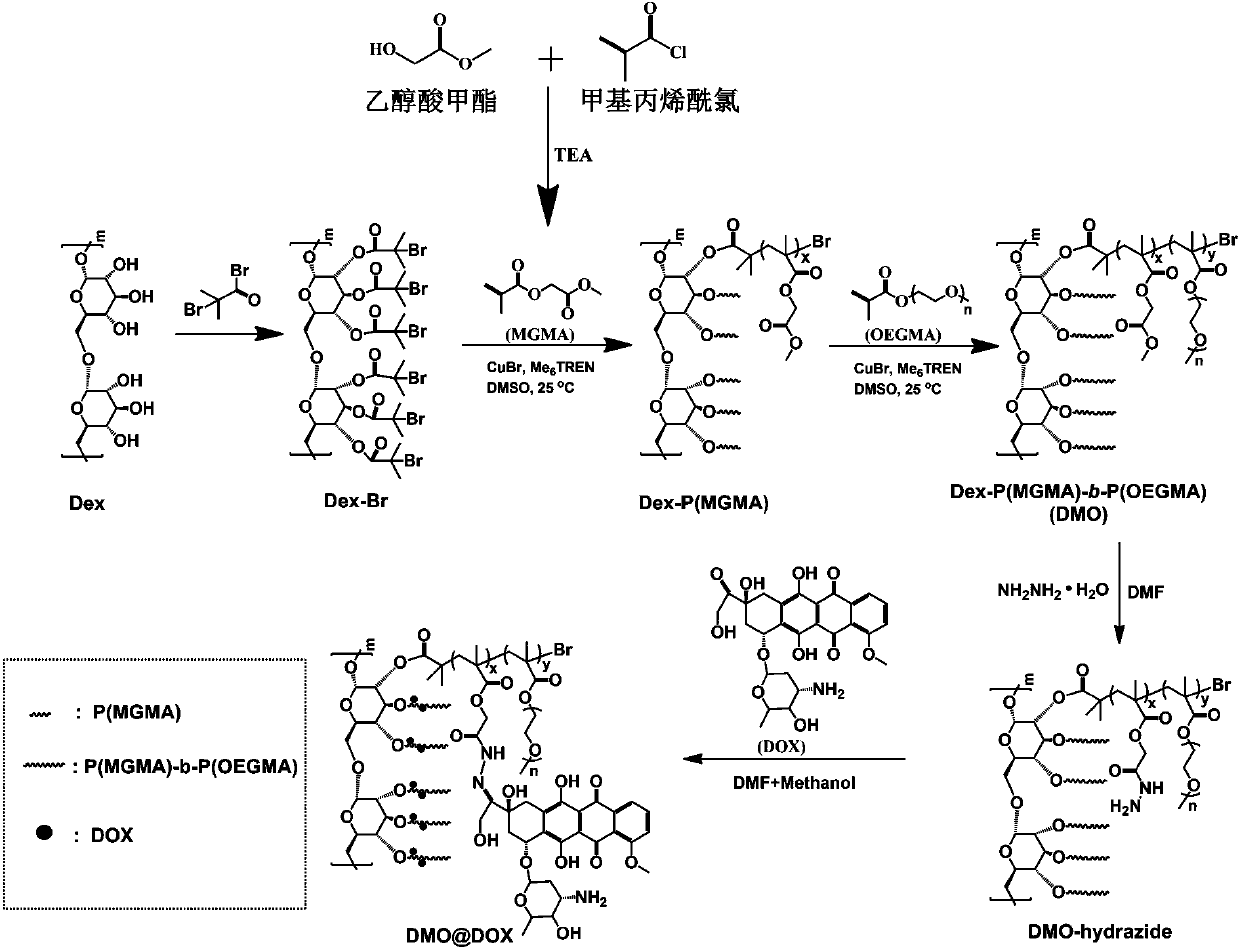
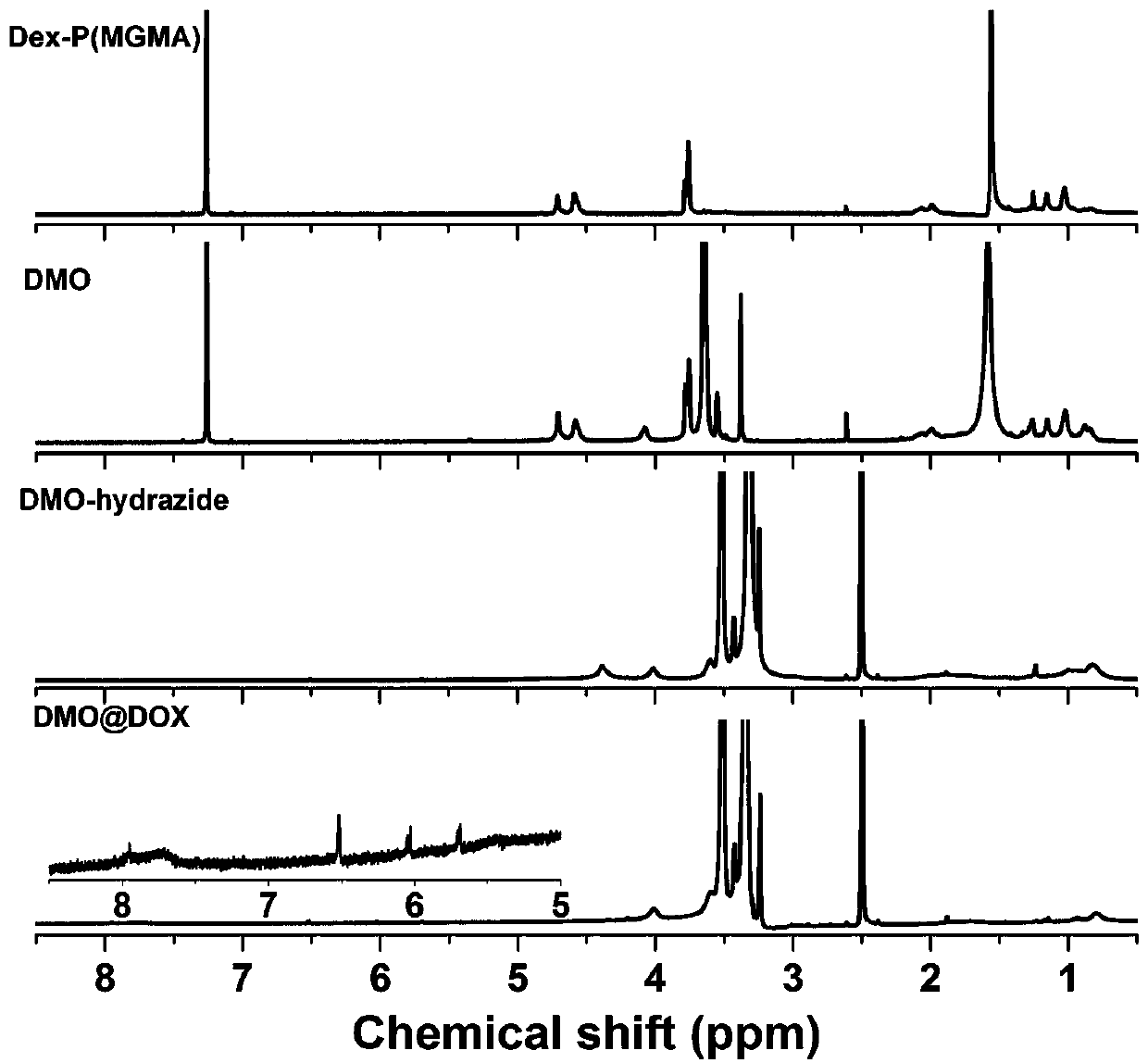
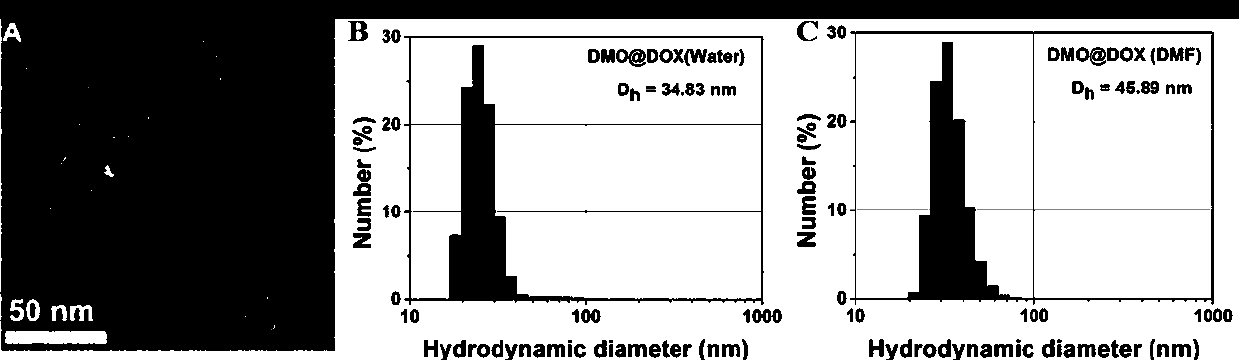
[0040] Example 1 Preparation of pH-responsive Dex-P(MGMA)-b-P(OEGMA) amphiphilic rod-like polymeric prodrugs
[0041] (1) Preparation of Dex-Br: under argon atmosphere, dextran (Dex) dissolved in ionic liquid (1-allyl-3-methylimidazole chloride) was cooled to 0 °C, and N was added. -Mixed solution of methylpyrrolidone (NMP) and N,N-dimethylformamide (DMF), then slowly add 2-bromoisobutyryl bromide (BIBB), ice bath for 0.5-2h, then warm to room temperature (25 ℃) and react in the dark for 12-72h, then precipitate in deionized water, dissolve the obtained precipitate with acetone, and repeat the purification three times to obtain a light yellow intermediate product, which is dried in a vacuum drying oven (25-30 ℃), and then the obtained The pale yellow intermediate product was dissolved in NMP, cooled to 0°C, then slowly added BIBB, ice bathed for 0.5-2h, then warmed to room temperature (25°C) and reacted in the dark for 12-72h, then precipitated in deionized water, washed with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com