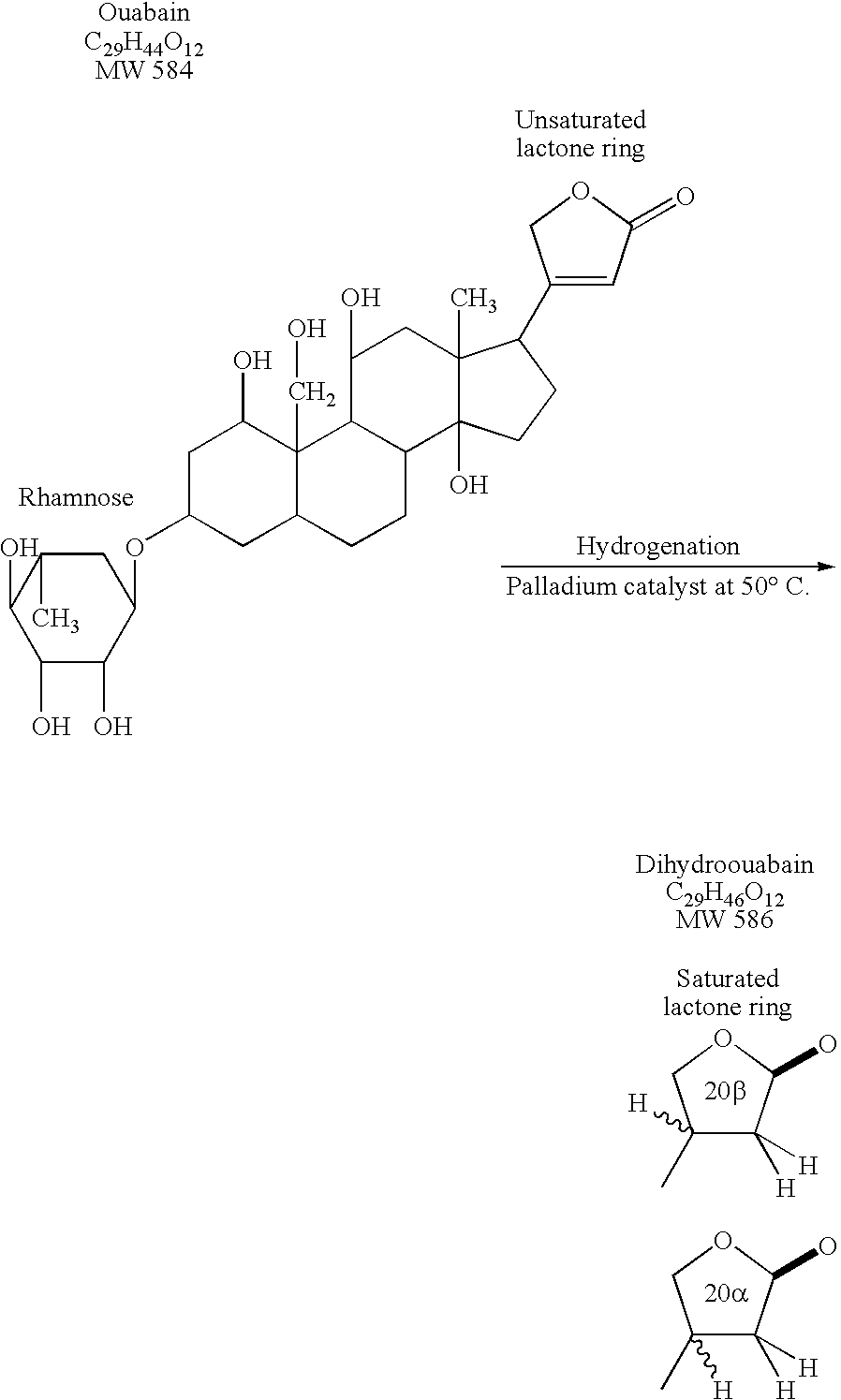
Dihydroouabain-like factor and diagnostic & therapeutic compositions and methods
a dihydroouabain-like factor and a technology of dh, which is applied in the field of mammalian ouabain-like factors (olf), can solve the problems of increased force and velocity of ventricular contraction, slow heart rate, and often accompanied by toxic effects that can be severe or lethal, and achieves lower potency, higher potency, and lower potency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Chemicals and Reagents
[0049] All chemicals used were reagent grade. 5-sulfosalicylic acid (SSA), calcium carbonate (CaCO3), digoxin, ouabain, ouabagenin, dihydroouabain, porcine cerebral cortex Na+,K+-ATPase (PCC), all reagents for catalytic inhibition of the sodium pump (adenosine 5′-triphosphate (ATP), ammonium molybdate, tween-80, bovine serum albumin (BSA), and all reagents for gel electrophoresis e.g., acrylamide, bis-acrylamide, N, N, N′, N′-tetramethyl-ethylene diamine (TEMED), Tris [Hydroxymethyl] aminomethane (TRIZMA base), sodium dodecyl sulfate (SDS), glycine, β-mercaptoethanol, bromophenol dye) except ammonium persulfate, which was purchased from Amresco (Solon, Ohio), were obtained from Sigma (St. Louis, Mo.). Digoxigenin and dihydrodigoxin were donated by Burroughs Wellcome Co. (Research Triangle Park, N.C.). Pre-stained protein markers were purchased from Bio-Rad (Richmond, Calif.). Phosphorus-32-phosphate (32 Pi, 1 mCi in 100 μL) was purchased from NEN, Life Science...
example 2
Equipment and Materials
[0050] A Polytron PT 3000 (Brinkman, Westbury, N.Y.) was used for homogenizing adrenocortical tissue and an Orion pH / SE meter model 710A (Orion, Cambridge, Mass.) for pH measurements. Solid phase extraction C-18 cartridges (Sep-Pak) were obtained from Waters Associates (Milford, Mass.). HPLC was performed on C-18 reverse-phase μ Bondapak columns (3.9×300 mm, 10-μm particle size) connected to a Waters 600E system controller and a Waters 966 photodiode array detector. Eluted fractions were collected with a Waters Fraction Collector from Millipore Corp. (Millford, Mass.) and evaporated with a Jouan Centrifugal Vacuum Concentrator RC 10.22 connected to a Jouan Refrigerated Trap RCT 60 (Winchester, Va.). For UV-spectrophotometry, we used a Hewlett-Packard (Palo Alto, Calif.) Model 8452A diode array spectrophotometer. A Beckman JA-2 centrifuge (Beckman, Palo Alto, Calif.) was used for centrifugation in the tissue preparation procedure. For sodium pump inhibition as...
example 3
[0053] Bovine adrenal glands were obtained from Pel-Freeze Biologicals (Rogers, Ark.) or provided by a local abattoir. The cortices were separated from medulla, sliced, chopped and homogenized in 2 ml dH2O g cortex using a Polytron PT 3000 homogenizer. The homogenate was centrifuged three times at 34,000 g for 30 minutes at 4° C. after which the pellet of each centrifugation step was resuspended in dH2O (1 ml / g cortex). The proteins in the tissue homogenates were precipitated by incubating the supernatant with 1% SSA at room temperature for 60 sec with continuous stirring followed immediately by adding an excess amount of CaCO3 until the pH increased and remained at 5.2. This extract was then centrifuged at 80,000×g for 10 min at 4° C. followed by vacuum filtration using two layers of Whatman #1 filter paper. Initial purification was performed by solid-phase extraction. The C-18 reversed-phase solid phase Sep-Pak extraction cartridges (Vac 10 cc) were primed with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com