Yeast fermentation small-molecular recombinant fibronectin protein peptide and preparation method and application thereof
A fibronectin and yeast fermentation technology, applied in the field of bioengineering, can solve the problems of low stability and low yield, and achieve the effects of good heat resistance, high yield and high degree of glycosylation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 constructs the expression plasmid encoding Chimeric FN protein
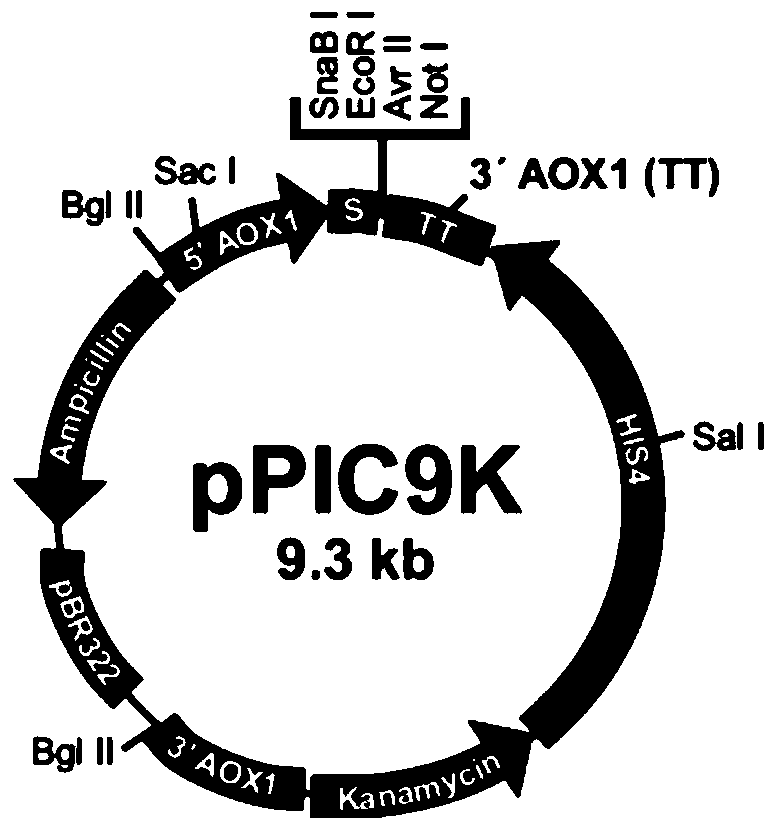
[0037] This embodiment uses the commercial carrier pPIC9K (such as figure 1 shown), purchased from Wuhan Sanying Biotechnology Co., Ltd., according to figure 1 Relevant sequence positions are designed to select restriction enzyme cutting sites EcoR I and Not I, and the gene sequence encoding Chimeric FN is artificially optimized with preferred codons of Pichia pastoris and obtained by artificial synthesis. The 5' and 3' ends of the synthesized recombinant fibronectin full-length DNA fragment each have a restriction endonuclease site, corresponding to EcoR I and Not I, respectively. The target fragment of recombinant fibronectin will be inserted between these two restriction sites to obtain the expression plasmid encoding Chimeric FN protein. Wherein, the amino acid sequence of recombinant fibronectin is:
[0038]ACSPPHSKSHCGGGGSIQWNAPQPSHISKYILRWRPKNSVGRWKEATIPGHLNSYTIKGLKPGVVYEGQL ISIQQYGH...
Embodiment 2
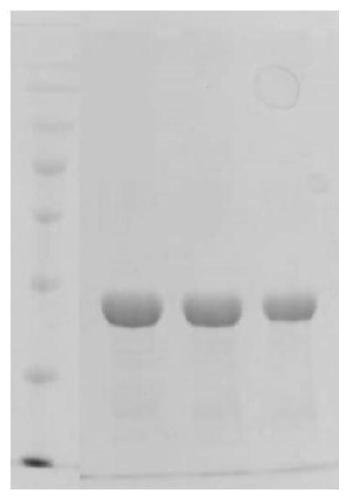
[0044] Embodiment 2 Expression purification and electrophoresis identification method of recombinant fibronectin
[0045] 1) Preparation of yeast clones. Genomic DNA of the expression vector Chimeric FN was extracted and digested with nuclease to obtain linearized DNA, which was dissolved in 5-10 ulTE (purchased from Neopro Biotech). Take 80ul of commercially competent Pichia pastoris GS115 (purchased from Tiangen Biology) mixed with 10ug of linearized DNA, and transfer it into a pre-cooled 0.2cm electroporation cup. Place on ice for 5 min. Set the machine parameters, immediately add 1ml of pre-cooled 1M sorbitol to the cup, transfer the contents to a sterilized centrifuge tube, divide it into 200ul aliquots, spread it on the MD plate, and incubate the plate at 30°C until the clones are produced, due to transformation The vector contains the mut gene, and only the successfully transformed strains can be screened by the mut phenotype, so the Mut+ / Muts phenotype strains are sc...
Embodiment 3
[0054] Example 3 Recombinant fibronectin promotes cell adhesion and growth test
[0055] The recombinant fibronectin purified in Example 2 was formulated into various concentrations (1, 6, 9, 15, 24 ug / ml), coated on a 96-well plate for 30 minutes, and washed twice with PBS. Add 1% BSA and block at 37°C for 30 minutes, add rat fibroblasts (cultivated with serum-free medium), gently suck the medium in the well after 1 hour, gently rinse the unadsorbed cells with PBS, and use CCD8 The number of living cells adsorbed on the bottom of the well plate was detected by the method to verify the activity of recombinant fibronectin. For the results, please refer to Figure 5 and Figure 6 .
[0056] Figure 5 The results showed that the cell attachment activity of the recombinant fibronectin was better than that of the natural structure fibronectin. Yeast-fermented proteins were significantly more effective in cell attachment than E. coli-fermented proteins due to the important role ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com