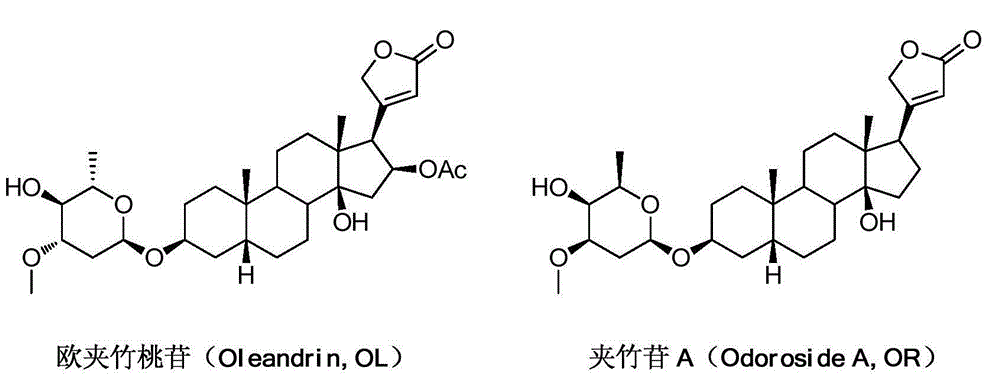
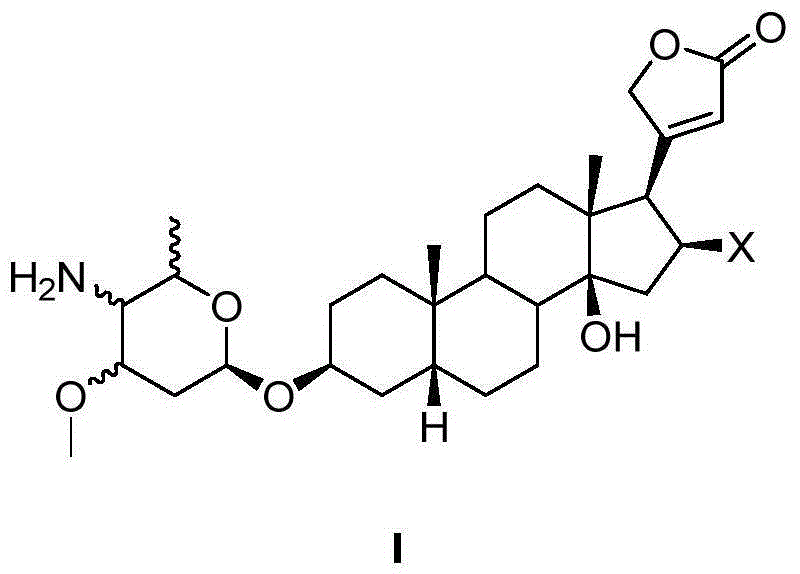
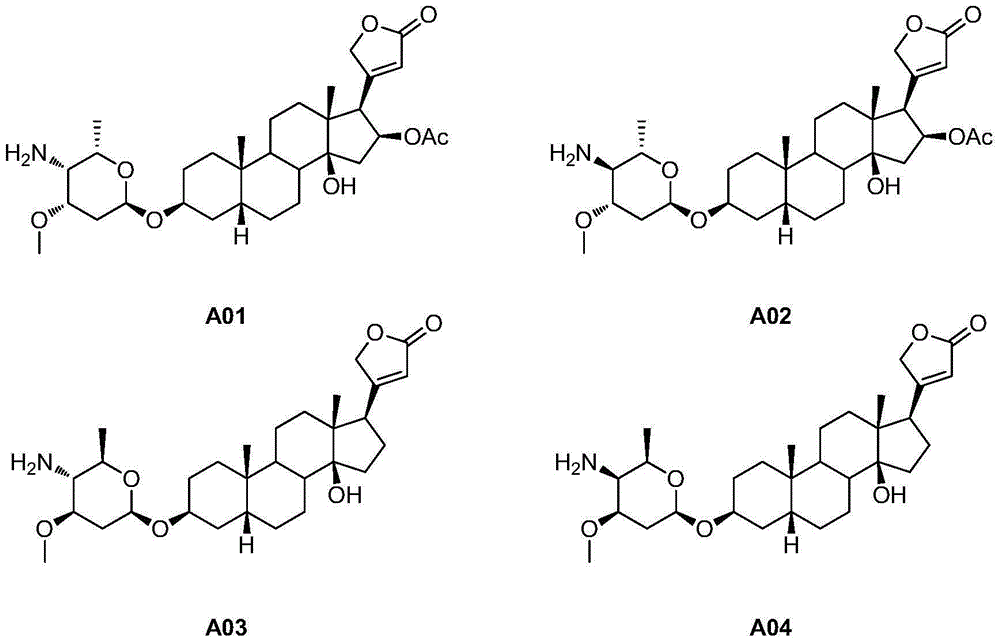
4'-amino-4'-dehydroxyl-oleandrin and 4'-amino-4'-dehydroxyl-odoroside A and use thereof
A pharmacy and compound technology, applied in the field of medicinal chemistry, can solve the problems of unpredictable anti-tumor activity, no clear correlation between anti-tumor activity and cardiotonic activity, etc., and achieves the effects of abundant synthetic raw materials, easy preparation and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: the extraction and separation process of compound OL and compound OR
[0048]
[0049] Take about 50kg of oleander leaves, add 40% ethanol for cold soaking, the soaking liquid is absorbed by D101 macroporous resin, and then eluted with 40%-70% ethanol / water gradient, collect the eluate containing OL and OR, elute After the solution was concentrated, the crude products of compound OL and compound OR were obtained by silica gel column chromatography, respectively. The crude product was recrystallized to obtain the pure products of compound OL and compound OR, which were used in the following reactions.
Embodiment 2
[0050] Embodiment 2: the synthesis of compound A01 and A02
[0051]
[0052] Compound OL (2.88 g, 5 mmol) was dissolved in dichloromethane (100 mL), and pyridinium chlorochromate (4.31 g, 20 mmol) was slowly added in portions. After the reaction solution was stirred and reacted at room temperature for 24 h, pyridinium chlorochromate (2.16 g, 10 mmol) was added, and the reaction was continued for 24 h. After the reaction, the reaction solution was diluted with dichloromethane (50mL), filtered through diatomaceous earth, the filtrate was concentrated, and intermediate B (2.70g, 94% ).
[0053] Intermediate B (2.70g, 4.7mmol) was dissolved in methanol (50mL), sodium acetate (771mg, 9.4mmol) and hydroxylamine hydrochloride (654mg, 9.4mmol) were added, and the reaction was stirred at room temperature for 2h. After the reaction, the reaction solution was concentrated under reduced pressure to remove methanol, dichloromethane (100 mL) was added to dilute, the reaction solution w...
Embodiment 3
[0057] Embodiment 3: the synthesis of compound A03 and A04
[0058]
[0059] Compound OR (518mg, 1mmol) was dissolved in pyridine (3mL), and p-toluenesulfonyl chloride (381mg, 2mmol) was slowly added dropwise under ice-bath stirring. After the dropwise addition, the temperature was raised to 60°C and stirred overnight. After the disappearance of the starting material was detected by TLC, sodium azide (130 mg, 2 mmol) was added to the reaction solution, and the stirring reaction was continued for 3 h. After the reaction, the reaction solution was diluted with dichloromethane (20mL), washed twice with water, washed with saturated brine, and the dichloromethane layer was dried over anhydrous sodium sulfate, concentrated under reduced pressure and subjected to column chromatography (4:1, petroleum ether / acetone) to obtain intermediate D1 (326mg, 60%).
[0060] Intermediate D1 (326mg, 0.6mmol) was dissolved in tetrahydrofuran / water (8mL, 8:1), triphenylphosphine (1.69g, 6mmol) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com