Cardiac glycoside compounds and antitumor application thereof
A compound, cardiac glycoside technology, applied in the field of medicine, can solve problems such as less toxic anticancer drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: dark elimination vine ( Streptocaulon juventas ) Extraction and separation of cardiac glycosides:
[0039] dark wilting vine ( Streptocaulon juventas ) roots (10kg) were extracted twice with 60% ethanol under reflux, each time for 1.5h, the extracts were combined, and the ethanol was recovered under reduced pressure until there was no alcohol smell. Disperse the ethanol extract in water to form a suspension, adjust the density to 1.07, load the sample on the macroporous adsorption resin (HPD100) column chromatography, elute with 20% ethanol, 55% ethanol, and 90% ethanol successively, and collect 55% ethanol The eluate was concentrated to obtain a fraction eluted with 55% ethanol (145 g). The eluted part was separated by silica gel column chromatography, eluted with dichloromethane: methanol system, and divided into 5 fractions A-E, of which fraction B (9.5g) was passed through a silica gel column (dichloromethane: methanol 15:1), reverse phase Compoun...
Embodiment 2
[0040] Embodiment 2: Malian saddle ( Streptocaulon griffithii ) Extraction and separation of cardiac glycosides:
[0041] Ma Lian saddle ( Streptocaulon griffithii ) roots (10kg) were extracted twice with 60% ethanol under reflux for 1.5 hours each time, the extracts were combined, concentrated, and dried to obtain the extract. The extract was dissolved in water, extracted three times with an equal volume of dichloromethane, then extracted three times with n-butanol, collected the n-butanol extract, and concentrated to obtain the n-butanol layer extract (92 g). The extract was divided into 5 fractions H-L through a silica gel column (ethyl acetate-methanol), fraction I (10g) was passed through a silica gel column (dichloromethane:methanol 15:1), reversed-phase silica gel column (50% methanol-water ) and recrystallization to obtain compound 1 and sub-fraction I1. I1 was purified by preparative HPLC with 55% methanol as the mobile phase to obtain compounds 9 and 12. Fracti...
Embodiment 3
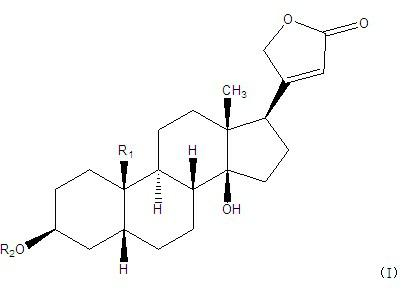
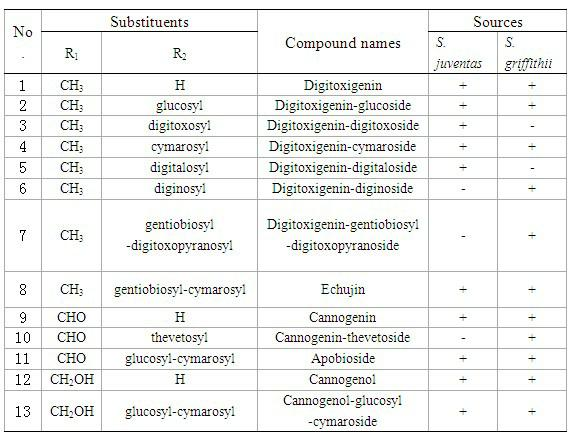
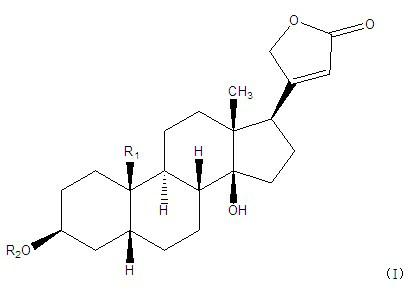
[0043] Embodiment 3: in embodiment 1 and embodiment 2, have the in vitro tumor inhibition experiment of the cardiac glycoside compound of general formula (I):
[0044] Cardiac glycoside compounds 1-13, compounds 7 and 11 compositions, and compound 1-13 mixed in equal proportions were tested by MTT method on Hela cervical cancer cells, HT29 human colon cancer cells, and A549 human lung adenocarcinoma cells. , SUP-B15 acute lymphoblastic leukemia cells, SGC7901 human gastric cancer cells, and Huh-7 human liver cancer cells in vitro tumor inhibitory activity (results are shown in Table 5). The activity test method is as follows:
[0045] The experiment set up a negative control group (water), a DMSO solvent control group, a positive control group (paclitaxel) and 5 different concentrations (0.01, 0.1, 1, 5, 1 0μm) of samples to be tested (compounds 1~13, 7 and 11 composition, a composition mixed in equal proportions of 1 to 13). Hela, HT29, A549, SUP-B15, SGC7901, and Huh-7 tum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com