Blank liposome taking ginsenoside Rg3 or analogue thereof as membrane material, and preparation method and application thereof
A technology of blank liposomes and ginsenosides, applied in the direction of liposome delivery, glycoside steroids, organic chemical methods, etc., can solve the problems of poor water solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
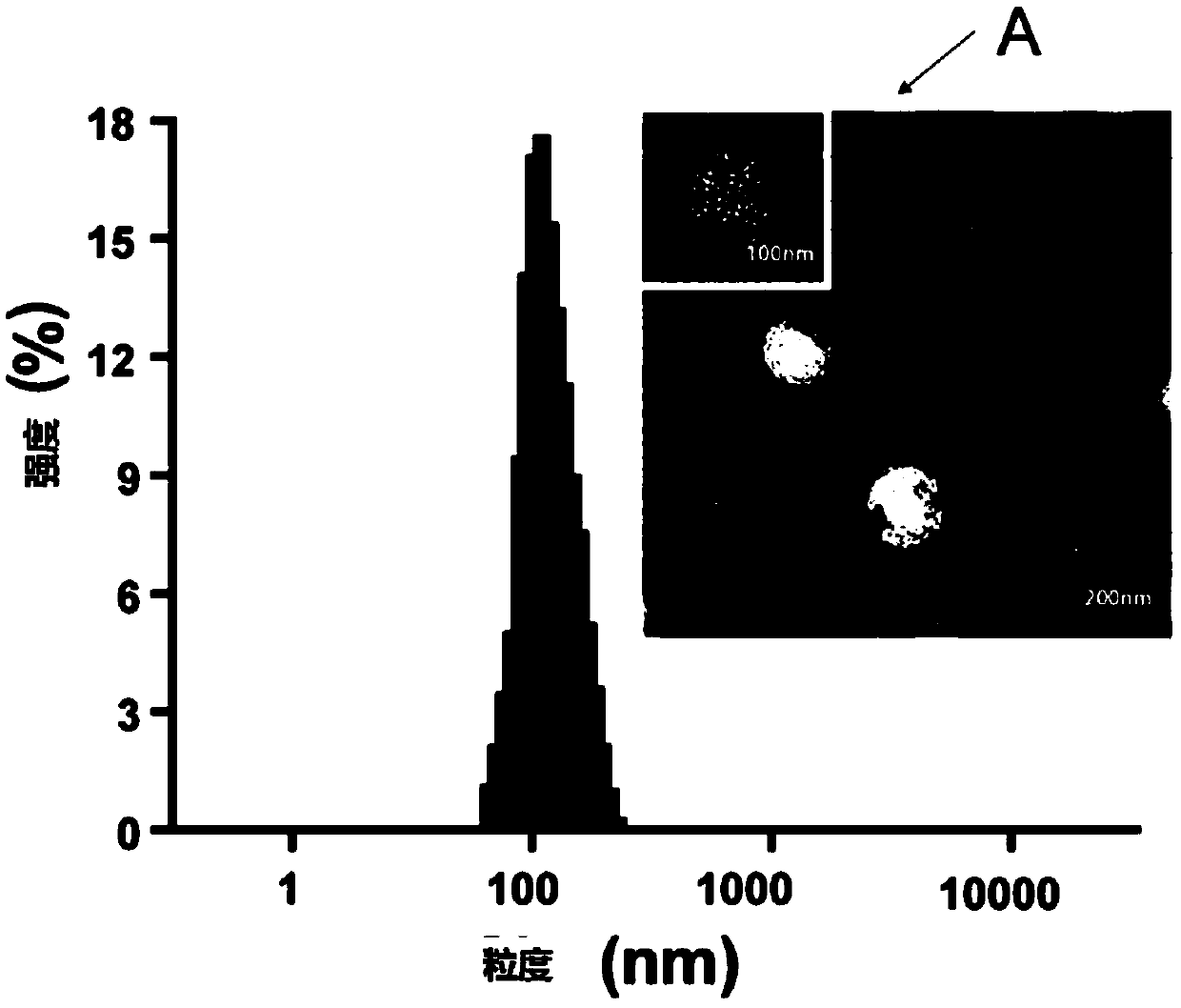
[0135] The preparation of embodiment 1 common Rg3 liposome
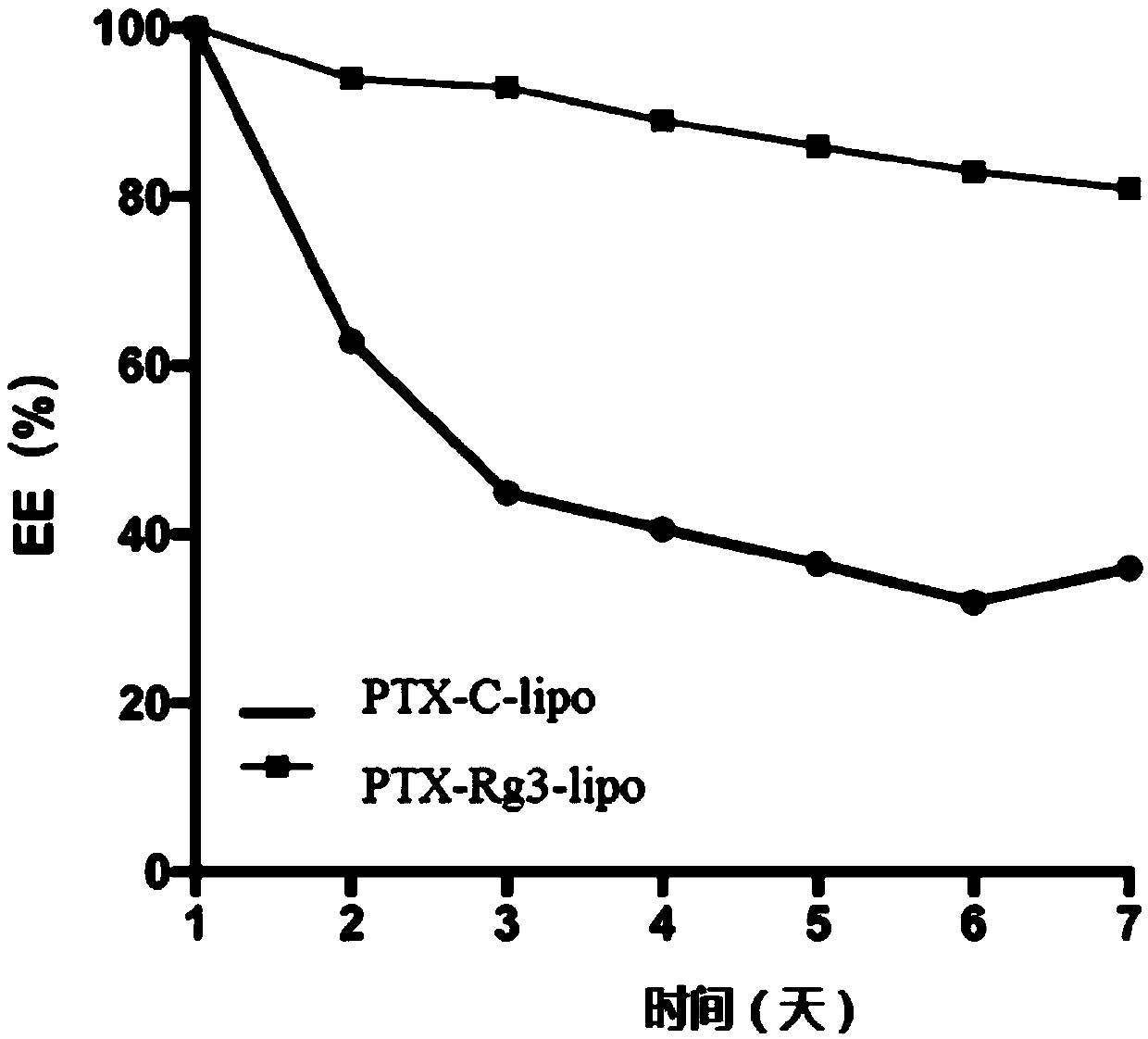
[0136] Add 1g of egg yolk lecithin, 0.1g of cholesterol, and 0.1g of ginsenoside 20(S)-Rg3 (without micronization treatment) into 20mL of absolute ethanol, and stir at room temperature to form a clear solution. In a constant temperature water bath at ℃, remove the organic solvent by rotary evaporation, and form a film, add 20mL of 5% trehalose aqueous solution (the stated percentage refers to the percentage of the quality of trehalose in the total mass of trehalose aqueous solution), and sonicate until the liposome particles 0.1-0.3 micron, pass through a microporous membrane of 0.22 micron to obtain an aqueous solution containing ginsenoside Rg3 liposome, and then put the solution in a vial. Put the above aqueous solution into a freeze dryer to freeze dry for 72 hours, pass through a protective gas (argon or nitrogen), seal it, and obtain the common ginsenoside Rg3 liposome. After testing, the D10 of the liposome w...
Embodiment 2
[0137] The preparation of embodiment 2 Rg3 blank liposomes
[0138] Add 1g of egg yolk lecithin and 0.1g of superfine powdered ginsenoside 20(S)-Rg3 into 200mL of chloroform, stir at room temperature to form a clear solution, and remove the organic solvent by rotary evaporation in a constant temperature water bath at 40-50°C. To form a film, add 20mL of 5% trehalose aqueous solution (the percentage refers to the percentage of the quality of trehalose in the total mass of trehalose aqueous solution), sonicate until the liposome particles are at 0.1-0.3 microns, and pass through a 0.22 micron microporous membrane , to obtain an aqueous solution of blank liposomes containing ginsenoside Rg3, and then the solution is divided into vials. Put the above aqueous solution into a freeze dryer to freeze dry for 72 hours, pass through a protective gas (argon or nitrogen), seal it, and obtain the ginsenoside Rg3 blank liposome. After testing, the D10 of the liposome was 66nm, the D50 was ...
Embodiment 3
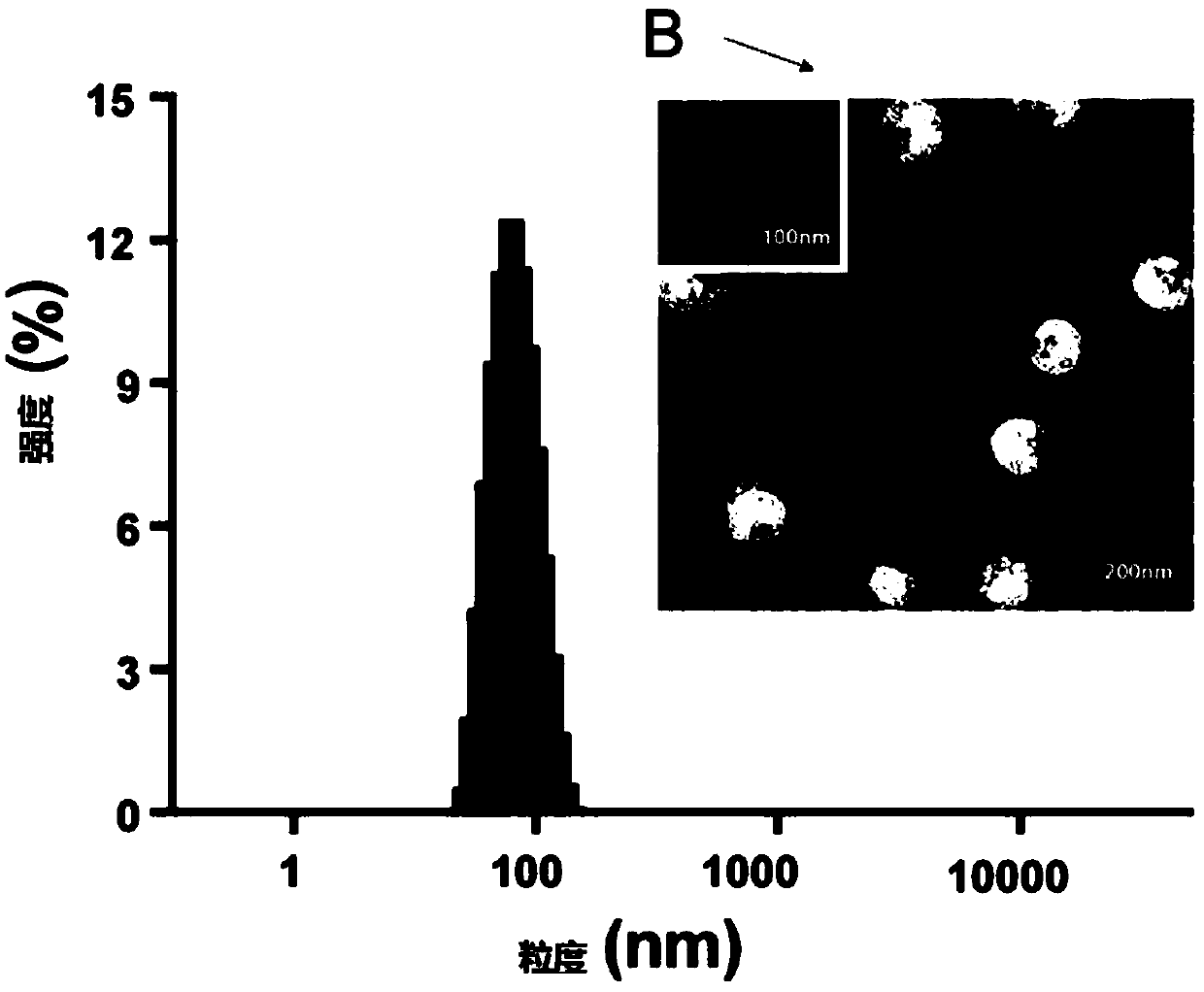
[0139] The preparation of embodiment 3 Rg5 blank liposomes
[0140] By the same method as in Example 2, Rg3 was replaced by Rg5 to prepare Rg5 blank liposomes. After testing, the D10 of the liposome was 70nm, the D50 was 96nm, and the D90 was 111nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com