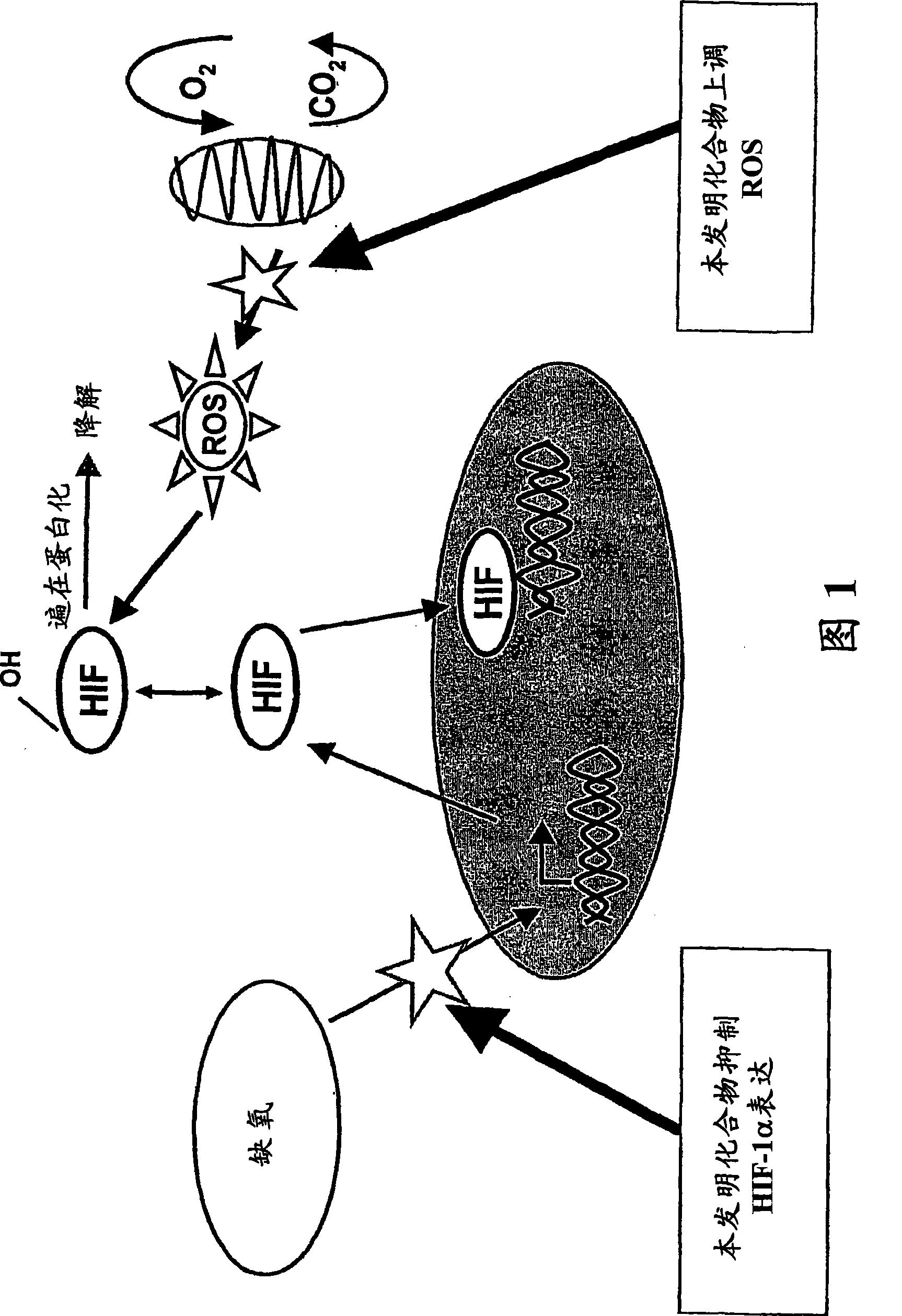
Modulators of hypoxia inducible factor-1 and related uses
A technology of R12 and R16, applied in the field of cardiac lactone and bufadienolide compounds, can solve the problem of not yet clear hypoxia overall regulator and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0216] Example 1: Cardiac glycoside compounds inhibit the expression of HIF-1α
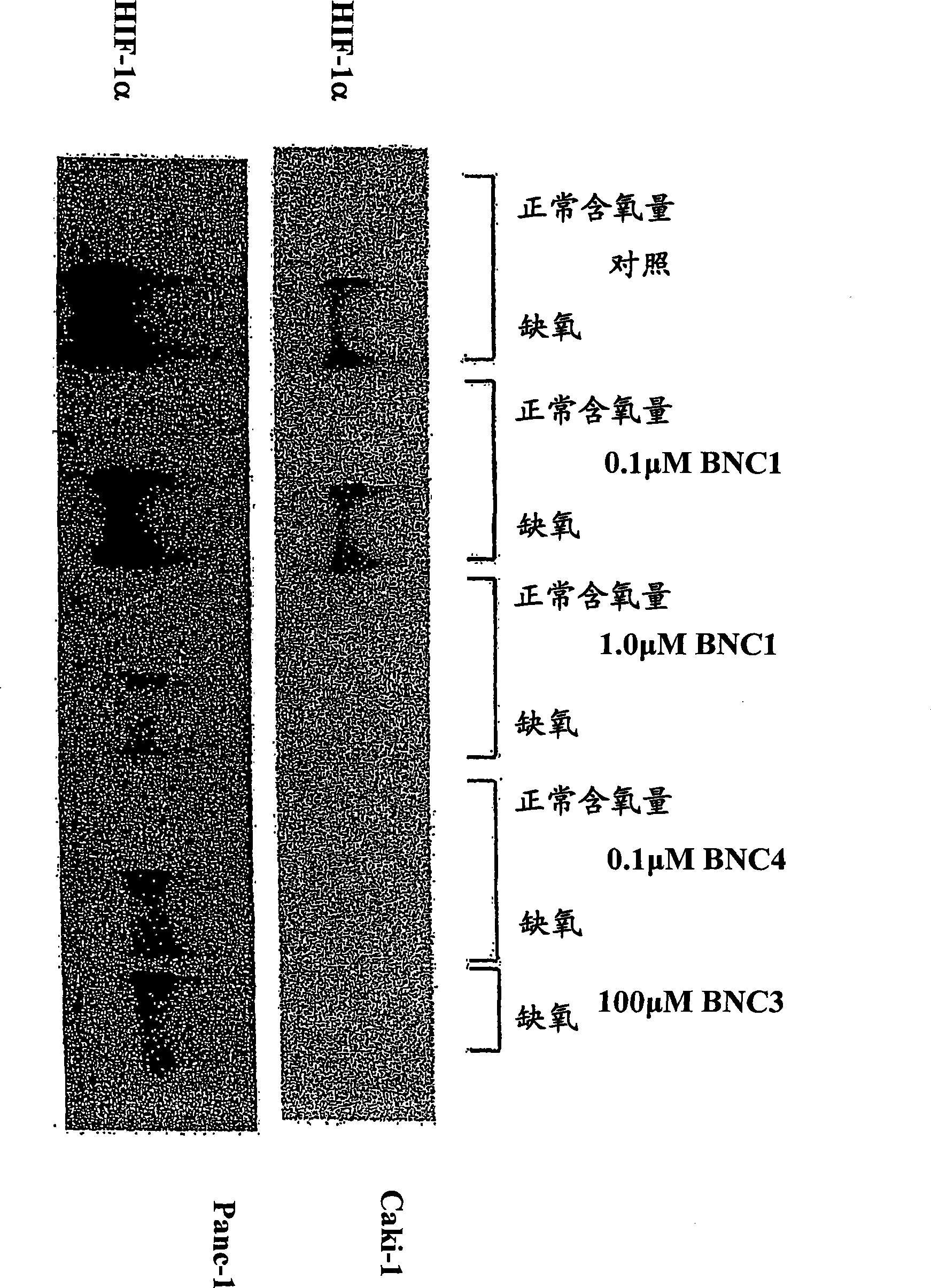
[0217] The ability of BNC1 and BNC4 to suppress hypoxia-mediated induction of HIF1α in human tumor cells was investigated. figure 2 Shown are the results of immunoblotting of HIF-1α, HIF-1β and β-actin (control) expression in Caki-1 or Panc-1 cells treated with BNC1 or BNC4 under hypoxia. The results showed that BNC4 was about 10-fold more active in suppressing HIF-1α expression than BNC1.
Embodiment 2
[0218] Example 2: BNC4 inhibits PHD inhibitor-induced HIF-1α under normoxia
[0219] In order to study the mechanism of BNC4 inhibiting HIF-1α, the ability of BNC1 or BNC4 to inhibit the expression of HIF-1α induced by PHD inhibitor L-mimosone (mimosone) was studied under normoxic conditions.
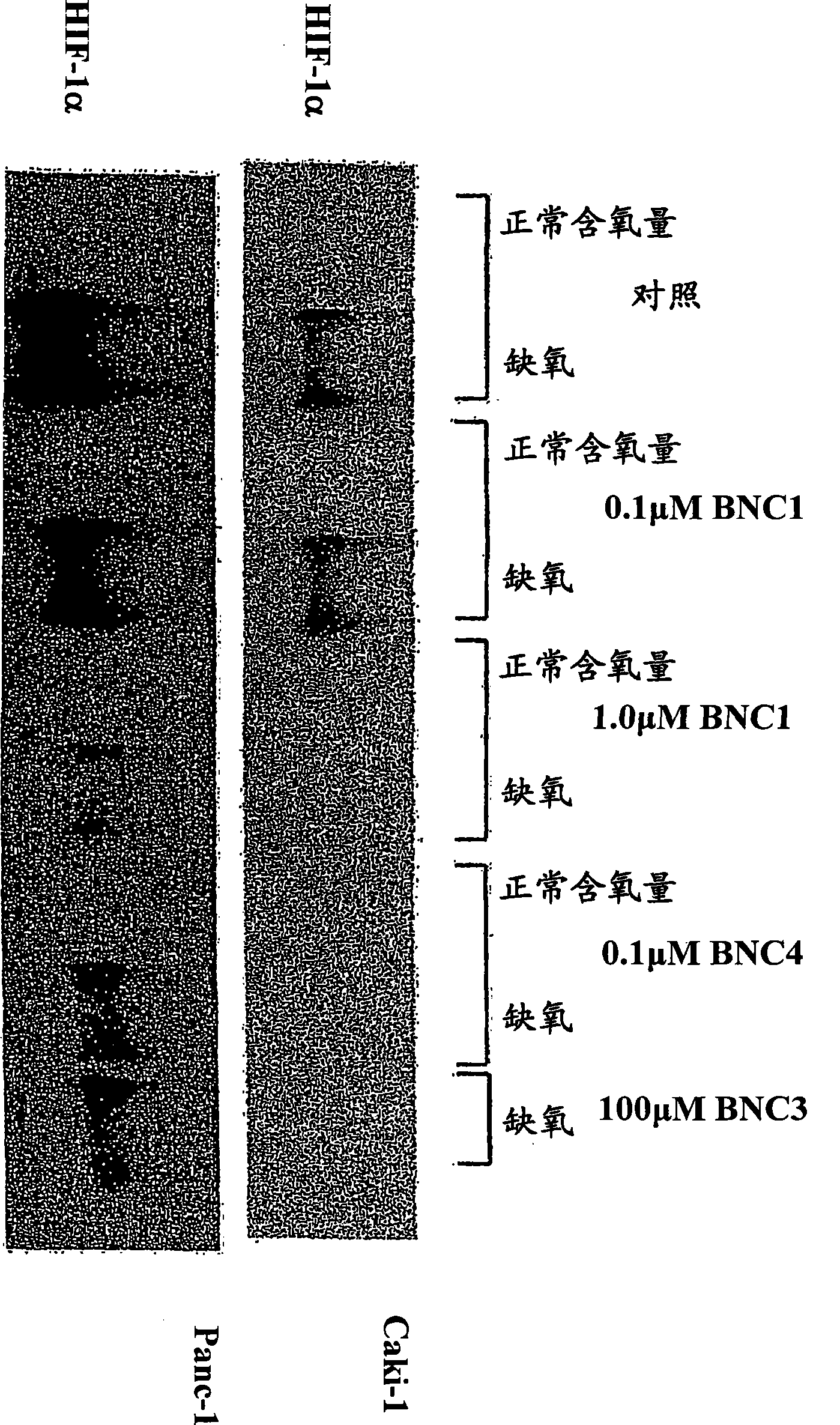
[0220] exist image 3 In a representative experiment, Hep3B cells were grown under normoxic conditions, but also treated with 200 [mu]M L-mimosolone for 18 hours in the presence or absence of BNC1 or BNC4 as indicated. The abundance of HIF1α and β-actin was determined by western blotting.
[0221] The results showed that under normoxic conditions, L-mimosoone induced the accumulation of HIF-1α, and the addition of BNC4 could abolish the L-mimosoone-induced HIF-1α accumulation. In this experiment, BNC1 at low concentrations tested appeared to have no effect on HIF-la accumulation. While not wishing to be bound by any particular theory, the fact that BNC4 inhibits PHD inhibitor-induced...
Embodiment 3
[0222] Example 3: Preparation of 3-oxime ethers and 3-amino derivatives of calcitonin
[0223] Synthesis of calicoin
[0224]
[0225] EtOH (1.25mL) solution (partial suspension)-0.02M acetate buffer (pH 4.0, 3.75mL) of insantrinin (66.3mg, 0.125mmol) and naringinase (23.2mg) was incubated at 40°C Incubate for 6.5h. After adding EtOH (30 mL), the whole mixture was concentrated under reduced pressure. The obtained residue was subjected to column chromatography (SiO 2 , 10 g, n-Hexane-EtOAc (1:1)) was purified to obtain indivalin (48 mg).
[0226] Synthesis of Scillarenon
[0227]
[0228] Dissolve 700mg (1.82mmol) calicoin in 30mL of anhydrous dichloromethane, add 1.4g molecular sieve powder and 1.57g (7.28mmol) pyridinium chlorochromate. The mixture was stirred overnight at room temperature under nitrogen atmosphere. The dark mixture was filtered through a pad of celite and concentrated. The crude mixture was purified by flash chromatography to afford 604 mg (86%)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com