Biological markers for evaluating therapeutic treatment of inflammatory and autoimmune disorders
a technology of inflammatory and autoimmune disorders and biological markers, applied in the field of human disease treatment, can solve the problems of limited applicability of biological markers in many patients, lack of accurate but simple methods for evaluating drug efficacy, and limited data available in vivo relating to other measurements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
Effect of Prednisone on Biological Markers of Atopy and Asthma
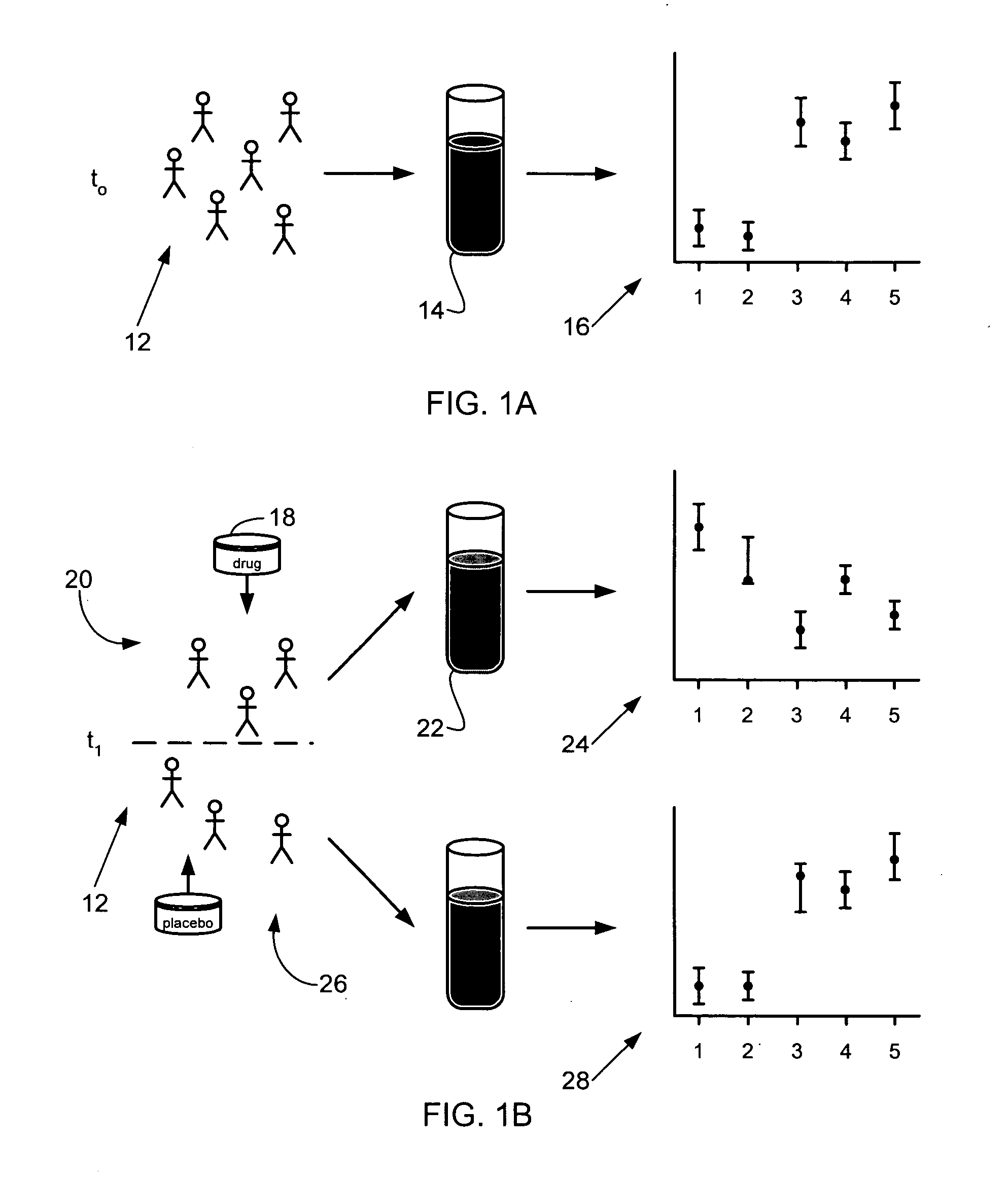
Methods
The inventive biomarkers were identified in a study of the effects of oral prednisone on biological markers of allergy and asthma. Eighty subjects were enrolled in the study, 23 with atopic asthma, 28 with allergy alone, 26 with neither allergy nor asthma (healthy controls), and 3 with asthma alone. Allergy was defined by a positive skin prick test. Mild asthma was defined as positive methacholine challenge within the last three months, as well as one or more of documented diagnosis of asthma, history of cough, recurrent wheeze, recurrent difficult breathing, and recurrent chest tightening. Approximately half of the subjects from each disease group were given oral prednisone for three days, while the other half was given a placebo. Blood samples were taken before treatment and after three days of treatment with oral prednisone or placebo.
Cellular markers in the blood samples were evaluated using the SurroScan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com