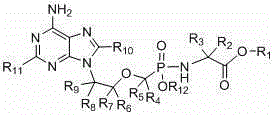
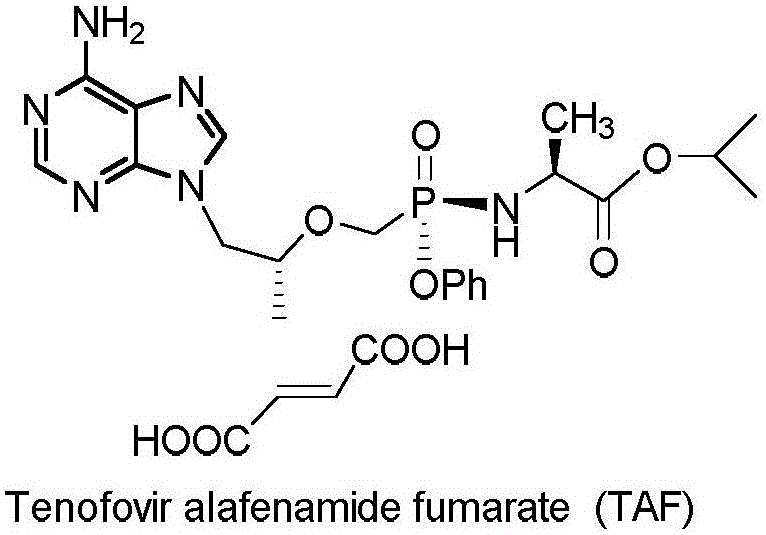
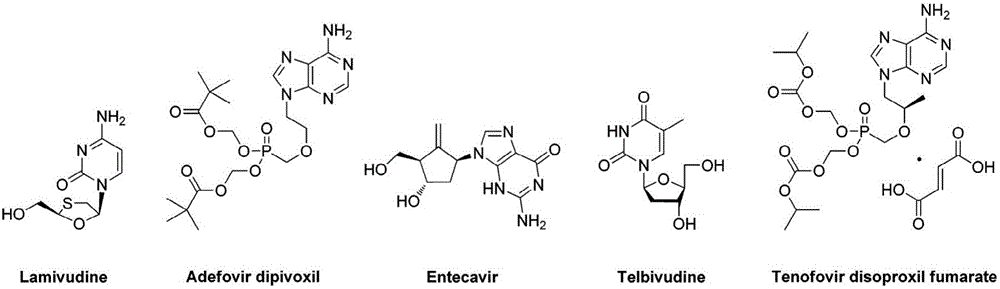
Preparation of non-cyclic nucleotide phosphoamides and salts thereof and application of non-cyclic nucleotide phosphoamides and salts thereof in aspect of antivirus
A technology of cyclic nucleotide phosphoramide and nucleotide phosphoramide, which is applied in the field of patients with or/and HBV infection, and can solve problems such as incomplete cure of drug resistance, side effects, and patient cessation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] step 1:
[0063] Isopropyl(tert-butoxycarbonyl)-L-alanine-3,3,3-d 3 (47)
[0064] L-Boc-alanine-3,3,3-d 3 (20 g, 0.104 mol) was dissolved in dry dichloromethane (400 mL), followed by the addition of isopropanol (6.87 g, 0.114 mol). The reaction solution was cooled to 0°C-5°C, and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCHCl) (30.02g, 0.156mol) and 4-di Methylaminopyridine (DMAP) (1.2g, 0.0104mol). The mixture was slowly raised to room temperature, stirred overnight, diluted with dichloromethane, and the organic phase was washed with saturated sodium bicarbonate and saturated brine, dried over anhydrous sodium sulfate, and concentrated , silica gel column chromatography (eluent: petroleum ether / ethyl acetate: 100 / 10) to obtain compound 47, 18.2g, 75%. 1 HNMR (400MHz, DMSO-d 6 ): δ7.24(d,1H), 4.90(m,1H), 3.92(m,1H), 1.14-1.37(m,15H). MS-ESI:235.32(M+H) + .
[0065] Step 2:
[0066]
Embodiment 2
[0083] The same method is used to synthesize phosphoramide D-amino acid isopropyl ester:
[0084]
[0085] Isopropyl((R)-((((R)-1-(6-amino-9H-purin-9-yl)propan-2-yloxy)methyl)(phenoxy)phosphoryl)- D-alanine-3,3-3-d 3 Fumarate (51)
[0086] Isopropyl((R)-((((R)-1-(6-amino-9H-purin-9-yl)propan-2-yloxy)methyl)(phenoxy)phosphoryl)- D-alanine 3,3-3-d 3
[0087] (3) (0.4g, 0.83mmol), add fumaric acid (88mg, 0.75mmol) to 9mL acetonitrile, reflux until all solids are dissolved, filter hot, cool to 5°C, stand at 5°C for 12 hours, filter , washed with 4 mL of acetonitrile to obtain 0.3 g of white solid, 61%. 1 HNMR (400MHz, DMSO-d 6 ):13.13(s,2H),8.11,8.14(2s,2H),7.02-7.34(m,5H),6.63(s,2H),5.59(m,1H),4.84(m,1H),3.83- 4.26(m,6H),1.04-1.15(m,9H). MS-ESI:480.5(M+H) + .
[0088] The following compounds and corresponding fumarates were synthesized by the same method:
[0089]
[0090] Reaction 2
[0091]
Embodiment 3
[0093] step 1:
[0094]
[0095] Propan-2-yl 2-d(tert-butoxycarbonyl)-L-alanine (53)
[0096] L-Boc-alanine (2.18 g, 11.5 mmol) was dissolved in dry dichloromethane (40 mL), followed by the addition of 2-deuteroisopropanol (0.98 mL, 12.6 mmol). The reaction solution was cooled to 0°C-5°C, and EDCHCl (3.31g, 17.2mmol) and DMAP (140mg, 1.15mmol) were added in batches. The mixture was slowly raised to room temperature, stirred overnight, diluted with dichloromethane, and the organic phase was washed with saturated Sodium bicarbonate, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated, and silica gel column chromatography (eluent: petroleum ether / ethyl acetate: 100 / 10) gave compound 53, 1.87g, 70%.1 HNMR (400MHz, DMSO-d 6 ): δ7.23(d,1H),3.91(m,1H),1.12-1.38(m,18H). MS-ESI:233.3(M+H) + .
[0097] Step 2:
[0098]
[0099] Propan-2-yl 2-d L-alanine hydrochloride (54)
[0100] Propan-2-yl 2-d(tert-butoxycarbonyl)-L-alanine (53) (1g, 4.3mmol) wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com