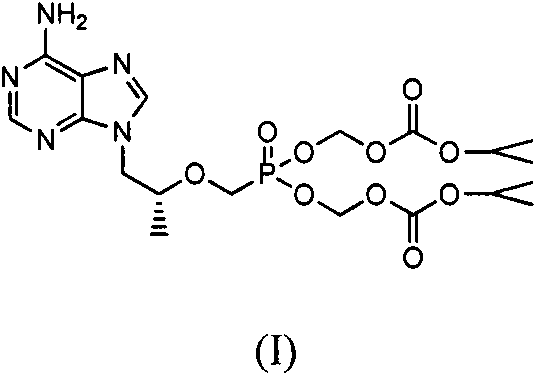
Preparation method of tenofovir disoproxil fumarate impurities
A technology of tenofovir disoproxil fumarate and tenofovir, applied in the field of preparation of tenofovir disoproxil fumarate impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
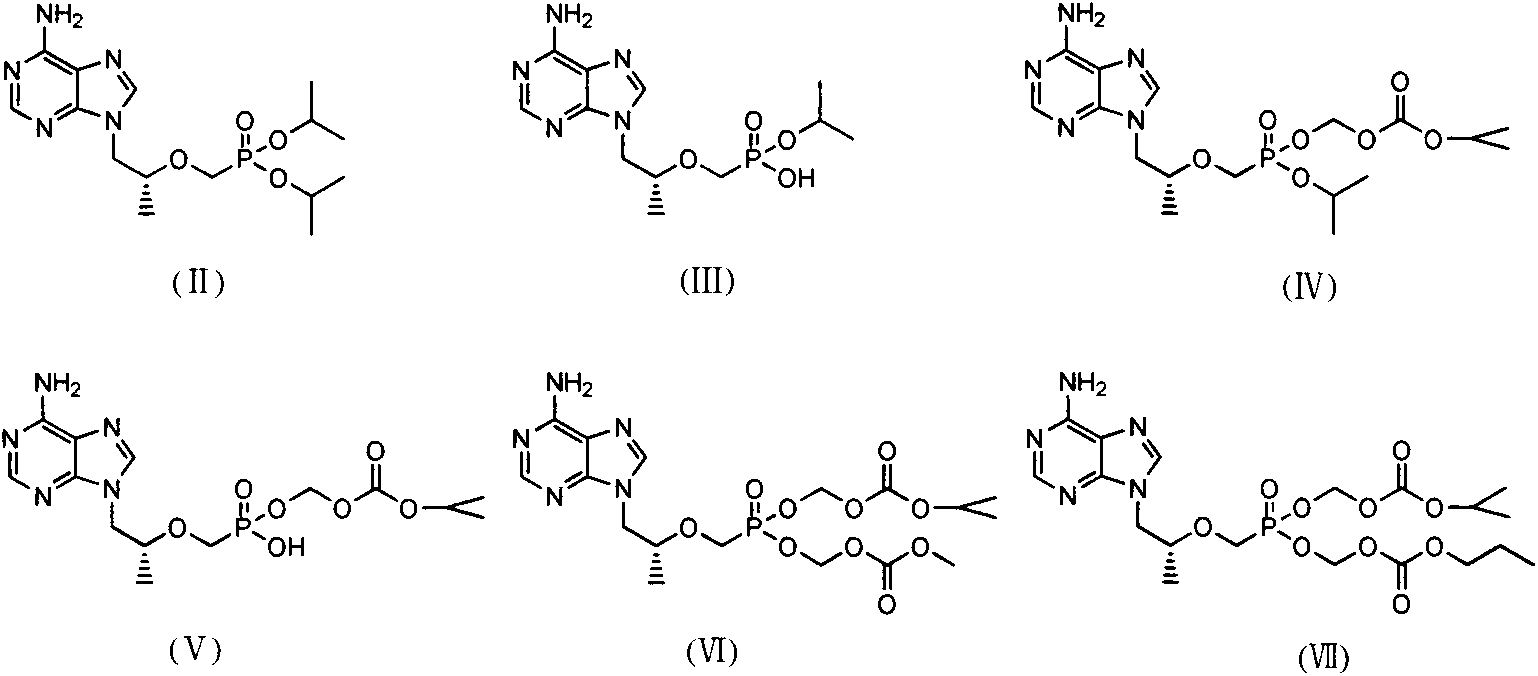
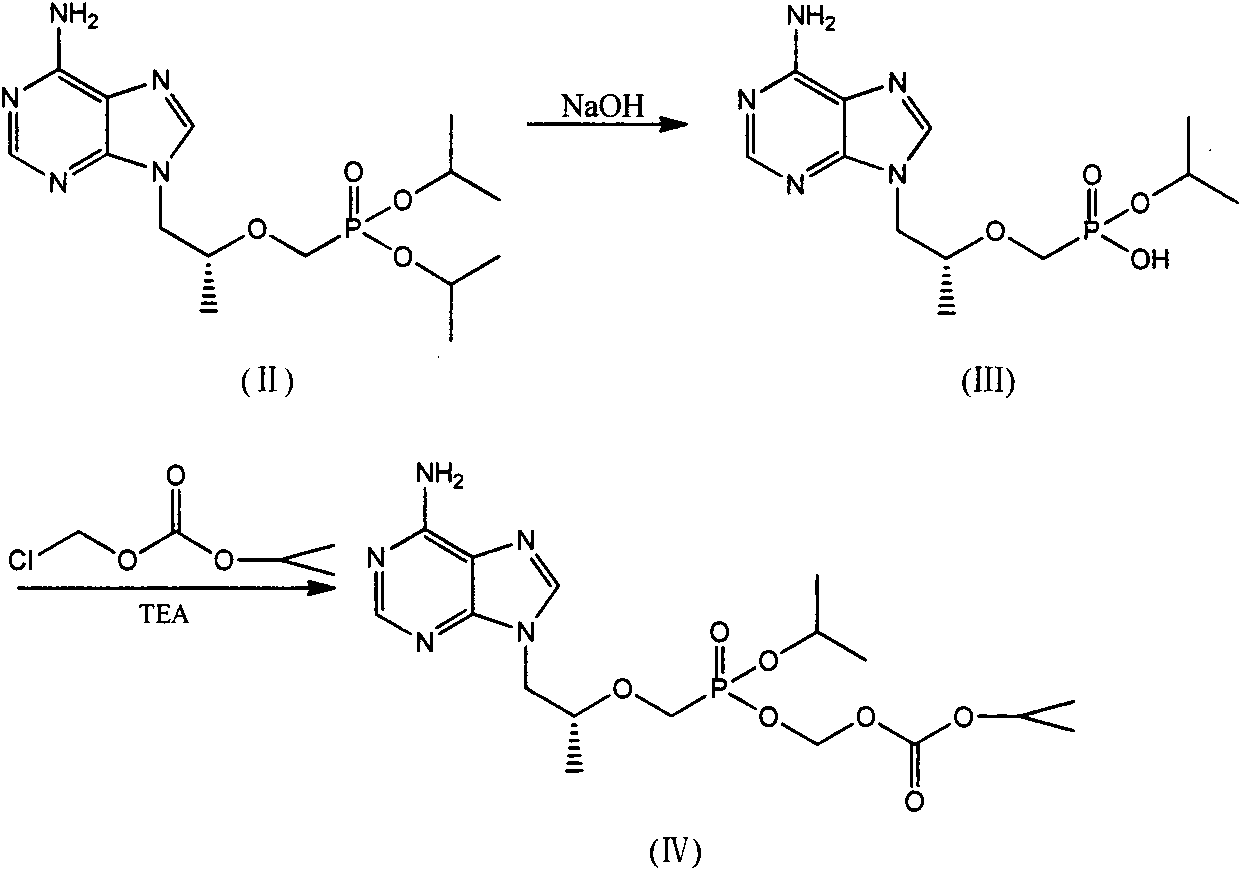
[0045] The preparation of embodiment 1 (R)-9-(2-phosphomethoxypropyl) adenine isopropoxycarbonyloxymethyl ester isopropyl ester (IV)
[0046] Put 3.7g of (R)-9-(2-methoxypropyl phosphate) adenine diisopropyl in a 100mL three-neck flask, add 40mL of water and 0.4g of sodium hydroxide at room temperature, and stir at room temperature for 8h. After the reaction was complete, it was concentrated, and the concentrate was separated by column chromatography to obtain 2.7 g of a white solid, with a yield of 82.8%.
[0047] Put 2.7g of the white solid obtained above into a 100mL three-necked flask, add 6.2g of chloromethyl isopropyl carbonate, 4.1g of triethylamine, 60mL of DMF at room temperature, heat and stir at 60°C for 8h, cool to room temperature, and add 30mL of acetic acid Ethyl ester was stirred for 30 min, separated, and the organic phase was washed with water and saturated NaCl aqueous solution, dried over anhydrous magnesium sulfate, filtered, and the concentrate was separa...
Embodiment 2
[0051] The preparation of embodiment 2 (R)-9-(2-phosphomethoxypropyl) adenine isopropoxycarbonyloxymethyl ester methoxycarbonyloxymethyl ester (VI)
[0052] Place 4g of (R)-9-(2-methoxypropyl phosphate) adenine mono(isopropoxycarbonyloxymethyl) ester in a 100mL three-neck flask, add 3.7g of methyl chloromethyl carbonate at room temperature , 3g triethylamine, 60mL DMF, heated and stirred at 60°C for 8h, cooled to room temperature, added 30mL ethyl acetate, stirred for 30min, separated, the organic phase was washed with water and saturated NaCl aqueous solution, dried over anhydrous magnesium sulfate, filtered and concentrated The product was separated by column chromatography to obtain 1.7 g of light yellow oil. Yield 34.8%.
[0053] Its structural identification data are as follows:
[0054] 1 H-NMR (400Mz, DMSO-d 6 )δ: 1.05 (d, J=6.4Hz, 3H, CH 3 ), 1.24(m, 6H, 2×CH 3 ), 3.77 (s, 3H, OCH 3 ), 3.93~4.03 (m, 3H, CHO, CH 2 P), 4.23 ~ 4.28 (m, 2H, CH 2 N), 4.78~4.85(m, 1...
Embodiment 3
[0056] The preparation of embodiment 3 (R)-9-(2-phosphomethoxypropyl) adenine isopropoxycarbonyloxymethyl ester n-propoxycarbonyloxymethyl ester (VII)
[0057] Place 4g of (R)-9-(2-methoxypropyl phosphate) adenine mono(isopropoxycarbonyloxymethyl)ester in a 100mL three-necked flask, add 4.6g of n-propyl chloromethyl carbonate at room temperature Esters, 3g triethylamine, 60mL DMF, heated and stirred at 60°C for 8h, cooled to room temperature, added 30mL ethyl acetate, stirred for 30min, separated, the organic phase was washed with water and saturated NaCl aqueous solution, dried over anhydrous magnesium sulfate, filtered, The concentrate was separated by column chromatography to obtain 1.9 g of light yellow oil. Yield 36.5%.
[0058] Its structural identification data are as follows:
[0059] 1 H-NMR (400Mz, DMSO-d 6 )δ: 0.87 (m, 3H, CH 3 ), 1.02~1.06 (d, J=6.4Hz, 3H, CH 3 ), 1.22~1.24(m, 6H, 2×CH 3 ), 1.56~1.65 (m, 2H, CH 2 ), 3.91~3.99 (m, 3H, CHO, CH 2 P), 4.00 ~ 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com