Preparing method for realizing industrial mass production of tenofovir disoproxil fumarate
A technology for tenofovir fumarate and dipyfurate, applied in the field of medicine, can solve the problems of unsuitable industrialized large-scale production, complex synthesis process, large environmental pollution, etc., and achieves easy purchase, high reaction conversion rate, and reduced The effect of pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
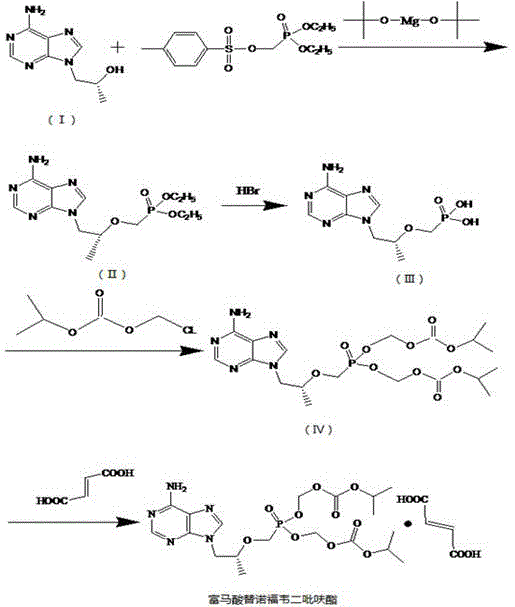
[0014] Example 1: (1) Preparation of (R)-9-[2-(diethoxyphosphonomethoxy)propyl]adenine
[0015] Add 100g of N,N-dimethylformamide, 30g of (R)-9-2-(hydroxypropyl)adenine, 8g of magnesium tert-butoxide and diethyl p-toluenesulfonyloxymethylphosphonate into the reaction vessel Stir 90g of the ester, heat to 90~95°C, keep warm and stir for 8 hours, terminate the reaction, and obtain the reactant. Cool to room temperature, add glacial acetic acid, adjust the pH to pH 6.5-7.5. Add an appropriate amount of dichloromethane, reflux for 5 hours, separate and extract the dichloromethane layer, filter, wash with dichloromethane, combine the filtrate and wash, and distill under reduced pressure to obtain a thick substance. The molar ratio of (R)-9-2-(hydroxypropyl)adenine to magnesium tert-butoxide is 1:0.6.
Embodiment 2
[0016] Embodiment 2: (2) tenofovir synthesis
[0017] Add (R)-9-[2-(diethoxyphosphonomethoxy)propyl]adenine into the reaction vessel, add 450 g of hydrobromic acid, stir, heat up and reflux for 10 hours, and the reaction ends. Distill under reduced pressure, cool to room temperature, add an appropriate amount of dichloromethane and water, separate and extract the dichloromethane layer, adjust the pH to 3.0 with 20% sodium hydroxide solution, lower the temperature to 10°C, crystallize for 12 hours, filter to obtain substitute Nofovir crude. Add an appropriate amount of water, heat until the solid dissolves, cool down to 10°C, conduct crystallization for 12 hours, filter to obtain tenofovir essence. Dry under reduced pressure at 55°C to obtain a dry product of tenofovir.
Embodiment 3
[0018] Embodiment 3: (3) Synthesis of tenofovir disoproxil
[0019] Add 300g of N-methylpyrrolidone, 90g of triethylamine and 25g of tenofovir into the reaction vessel, stir, heat to 70°C, add 40g of chloromethyl isopropyl carbonate dropwise, keep stirring for 10 hours, and stop the reaction . Add an appropriate amount of ethyl acetate, filter, wash the filter residue with an appropriate amount of ethyl acetate, combine the washed filtrates, add an appropriate amount of water to wash 3 times, add anhydrous sodium sulfate for dehydration, and filter. The filtrate was distilled under reduced pressure at 55°C and evaporated to dryness to obtain tenofovir disoproxil oil. The molar ratio of tenofovir and triethylamine is: 1:10.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com