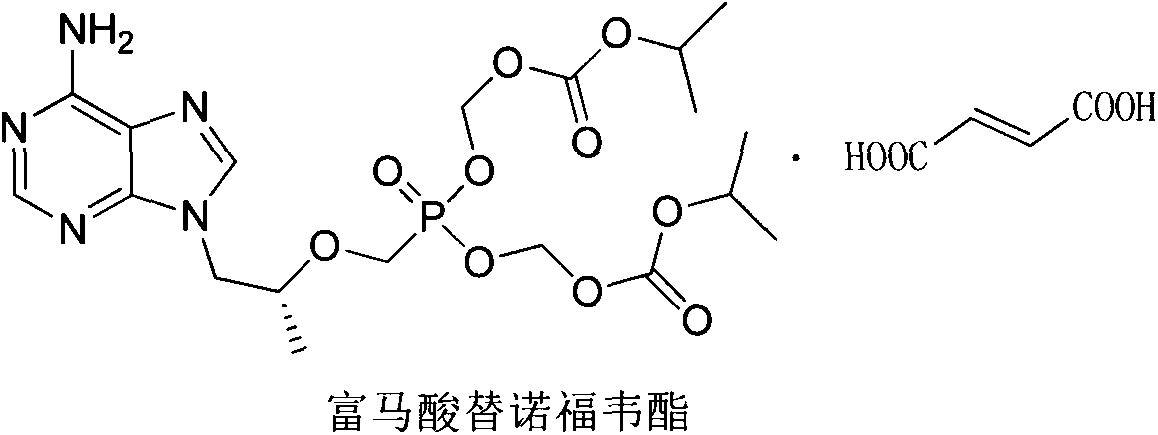
Preparation method of tenofovir disoproxil fumarate hemifumarate
A technology of tenofovir disoproxil and hemifumarate, which is applied in the field of preparation of tenofovir disoproxil hemifumarate, can solve the problems of time-consuming and labor-consuming, difficult industrial preparation, etc., and achieve convenient and reliable process operation control, simple and economical preparation method, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
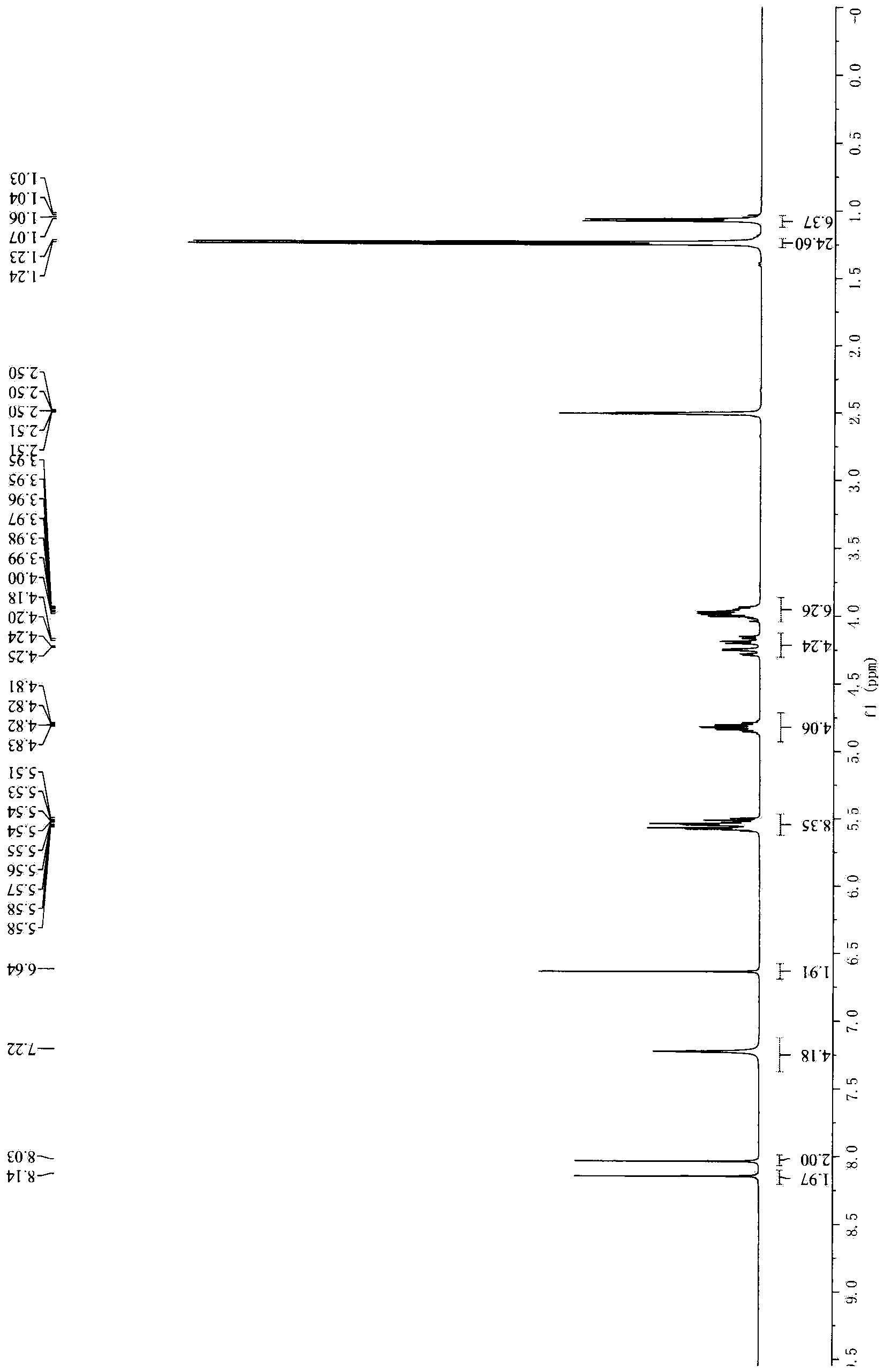
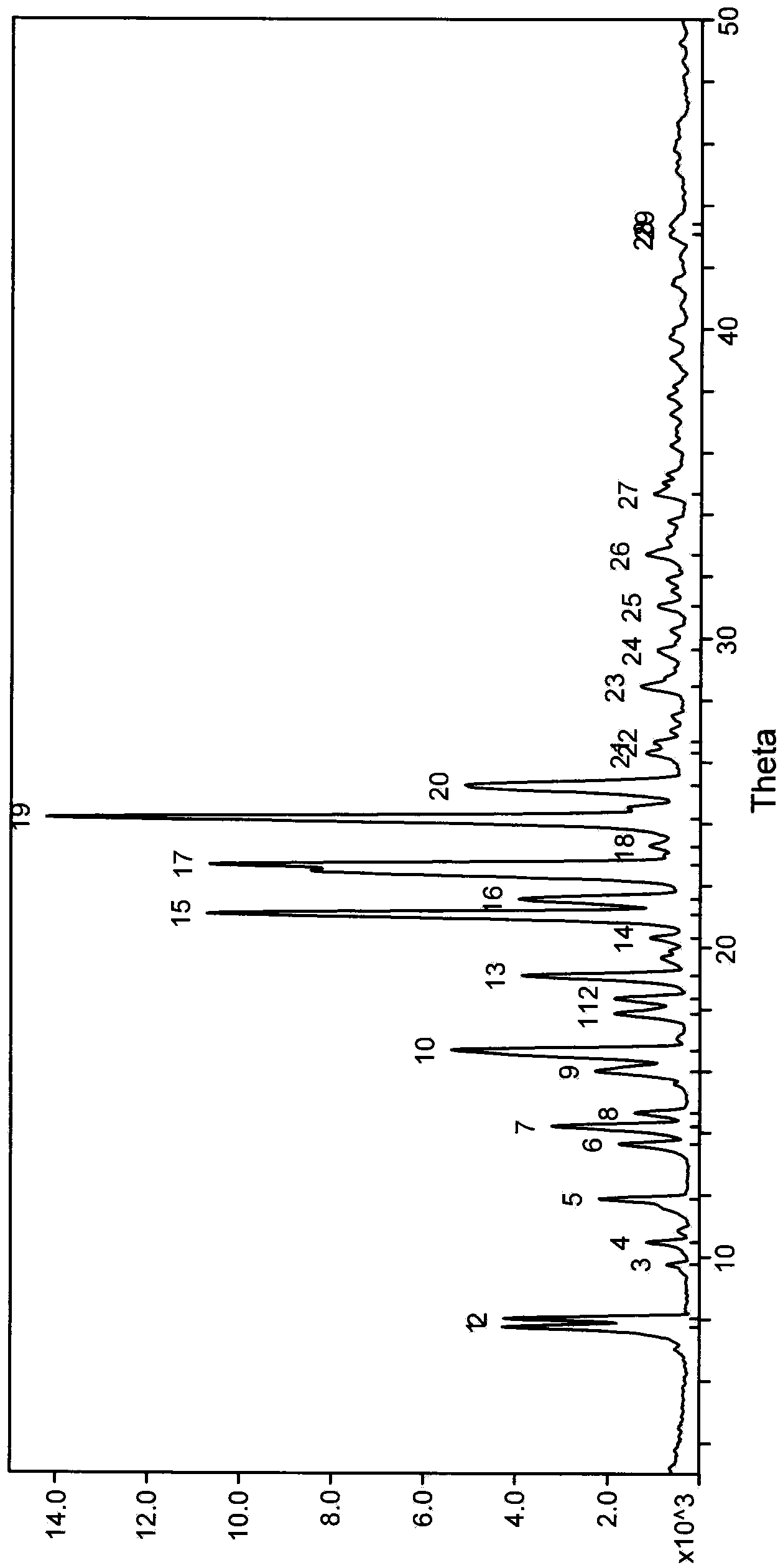
Image
Examples
Embodiment 1
[0017] Embodiment 1, the preparation of (R)-(+)-9-[2-(diethoxyphosphorylmethoxy)propyl]adenine
[0018] Add 40g DMF to a 250ml three-necked flask, add 12g (R)-(+)-9-(2-hydroxypropyl)adenine, add 11.3g magnesium tert-butoxide under stirring, stir, and heat up. The internal temperature was 70°C, and 31 g of diethyl p-toluenesulfonyloxyphosphonate was added dropwise to keep the internal temperature at 70°C. After the dropwise addition, stir for 5.0 to 6.0 hours, take samples and control in HPLC until the conversion of raw materials is complete. Cool down to 30-40°C, add 10g of acetic acid dropwise, keep the internal temperature at 30-40°C, and stir for 0.5-1.0h after the addition is complete. Concentrate the solvent under reduced pressure at 70-75°C until there is no distillation, and lower the temperature to 20-30°C. Add 150ml of dichloromethane, add 13g of water, and stir for 1.0-2.0h. Filtrate to obtain filtrate 1. The filter cake was beaten and washed with 60 ml of dichlo...
Embodiment 2
[0019] Example 2, Preparation of (R)-(+)-9-[2-(dihydroxyphosphorylmethoxy)propyl]adenine
[0020] Add the crude (R)-(+)-9-[2-(diethoxyphosphorylmethoxy)propyl]adenine from the previous step into a 250ml three-necked flask, add 30ml of acetonitrile, stir, and cool down. When the inner temperature is lower than 30°C, 37g of TMSBr is added dropwise to keep the inner temperature lower than 30°C. After the dropwise addition, raise the temperature, keep the internal temperature at 60-65°C, stir for 3.0-4.0 hours, take a sample by HPLC and control until the conversion of raw materials is complete, lower the temperature to 40-45°C, and concentrate the solvent under reduced pressure until there is no distillation. Cool down to 0-5°C, add 100g of purified water dropwise, keep at 0-5°C, stir for 1.0-3.0h, then extract twice with 50ml of ethyl acetate. Separate the water layer, control the internal temperature at 0-5°C, add dropwise 50% sodium hydroxide solution, adjust the pH value of t...
Embodiment 3
[0022] Embodiment three, the preparation of free tenofovir disoproxil
[0023] Add 50ml NMP in a 200ml nitrogen-protected three-necked flask, add 12.5g triethylamine, add 12.5g (R)-(+)-9-[2-(dihydroxyphosphorylmethoxy)propyl]adenine, Stir for 0.5~1.0h. Add 31.3g of chloromethyl isopropyl carbonate, raise the temperature to an internal temperature of 55-65°C, and stir for 5.0-6.0 hours to terminate the reaction. Cool down to 20-30°C, add 100ml cyclohexane and extract twice to obtain the lower layer. 60 ml of purified water was added to the lower layer mixture, 100 ml of ethyl acetate was added for extraction three times, and the organic phases were combined. Add water to the above organic phase and wash three times to obtain the organic phase, dry it with anhydrous sodium sulfate, filter, and concentrate to dryness under reduced pressure at 35-40° C. to obtain 20 g of free tenofovir disoproxil.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com