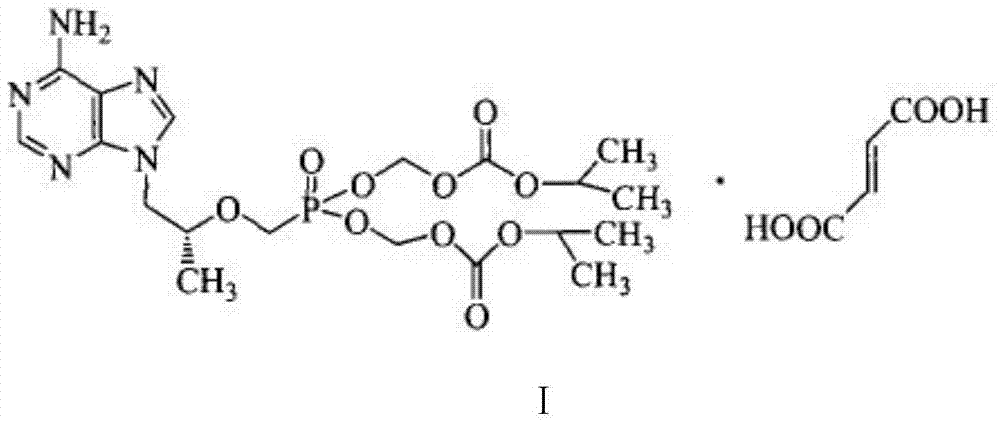
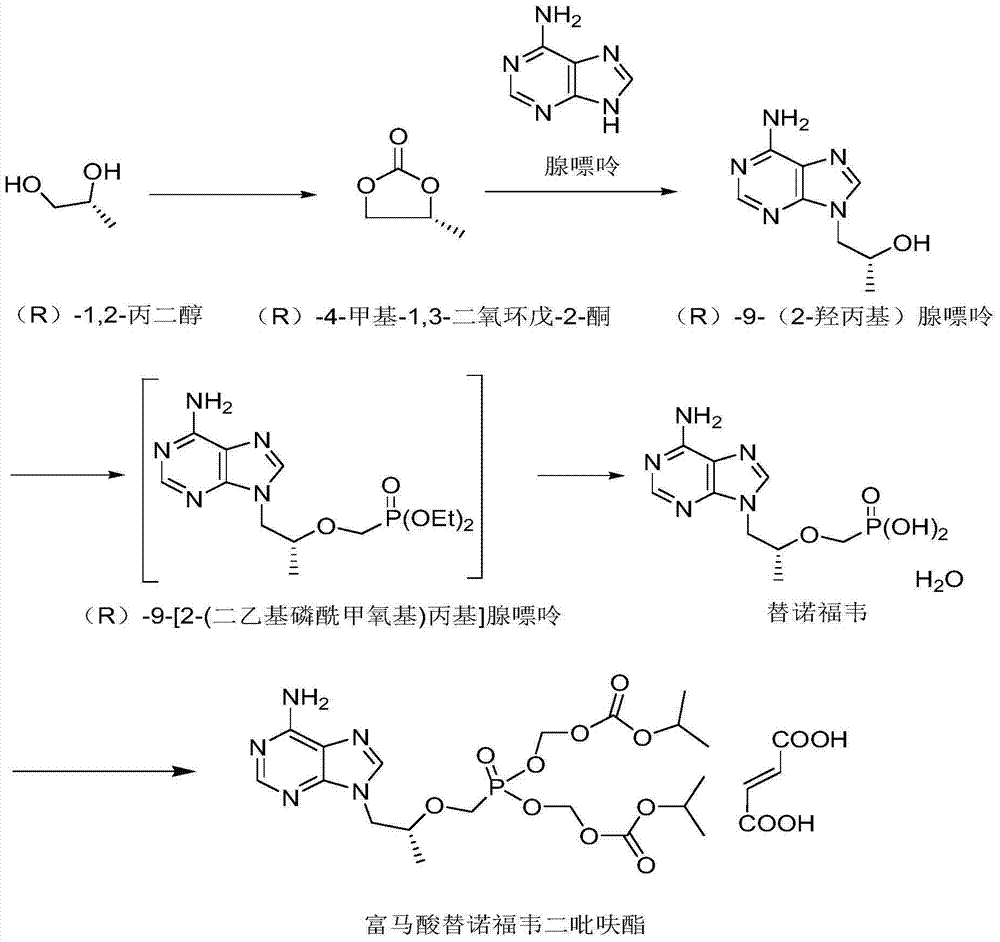
Tenofovir disoproxil fumarate synthesis method
A technology for tenofovir fumarate and dipyproxil, which is applied in the field of medicine, can solve the problems of low yield, complicated hydrolysis process, uneven mixing of materials, etc., and achieves improved purity and yield, good refining effect, Yield-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of (R)-4-methyl-1,3-dioxolan-2-one
[0033] 33.0g of (R)-1,2-propanediol, 1.2g of sodium methoxide, 76.9g of dimethyl carbonate, and 10ml of anhydrous methanol were added to a 200ml three-necked bottle. Turn on stirring, heat up to 80 ℃~90 ℃ and react, during which methanol in the reaction system is continuously evaporated, when basically no liquid flows out, TLC detects whether the reaction is complete [developing solvent is ethyl acetate (V / ml): petroleum ether (V / ml) = 3:2; iodine smoked color]. After the reaction is complete, heat up to 110°C to 120°C, continue to evaporate the solvent under reduced pressure at -0.09MPa, distill until no fraction flows out, and filter off the insoluble matter by suction filtration to obtain a pale yellow oil (R)-4-methyl. 40.8 g of base-1,3-dioxolan-2-one, with a purity of 98.7% and a molar yield of 92.1%. 1 HNMR (500MHz, CDCl 3): δ4.87~4.86 (m, 1H), 4.58~4.55 (m, J=8.5Hz, 1H), 4.05~4.02 (m, J=8.5Hz, 1H), ...
Embodiment 2
[0034] Example 2 Preparation of (R)-4-methyl-1,3-dioxolan-2-one
[0035] 33.0g (R)-1,2-propanediol, 0.8g sodium hydroxide, 51.3g diethyl carbonate, and 10ml absolute ethanol were added to a 500ml three-necked bottle. Turn on stirring, heat up to 85 ℃~95 ℃ and react, during the period, the absolute ethanol in the reaction system is continuously evaporated, and when basically no liquid flows out, TLC detects whether the reaction is complete [developing solvent is ethyl acetate (V / ml): Petroleum ether (V / ml) = 3:2; iodine smoked color]. After the reaction is complete, heat up to 110°C to 120°C, continue to evaporate the solvent under reduced pressure at -0.09MPa, distill until no fraction flows out, and filter off the insoluble matter by suction filtration to obtain a pale yellow oil (R)-4-methyl. 40.6 g of base-1,3-dioxolan-2-one, with a purity of 98.0% and a molar yield of 91.6%. 1 H NMR (500MHz, CDCl 3 ): δ4.87~4.86 (m, 1H), 4.58~4.55 (m, J=8.5Hz, 1H), 4.05~4.02 (m, J=8.5...
Embodiment 3
[0036] Example 3 Synthesis of (R)-9-(2-hydroxypropyl) adenine
[0037] Add 532ml of N,N-dimethylformamide, 40g of (R)-4-methyl-1,3-dioxolan-2-one, 47.2g of adenine and 0.85g of potassium hydroxide to a 2000ml three-necked bottle , turn on stirring, heat up to 135 ℃ ~ 145 ℃, keep the reaction for 22 hours, start TLC detection [developing solvent is dichloromethane (V / ml): methanol (V / ml) = 9:1], confirm that the reaction is complete Then, cool down to 80°C~90°C and begin to evaporate 4 / 5 of the reaction solvent under reduced pressure. After the evaporation is completed, add 672 ml of isopropanol, heat up to 70°C~80°C and stir to dissolve; after dissolving, open the refrigeration facility to cool down. Stir and crystallize at 0℃~5℃ for 1 hour, filter with suction, filter off the solvent, and vacuum dry the filter cake at -0.08MPa and 40℃ for 8 hours to obtain 57.7g of white solid, which is (R)-9-(2- Hydroxypropyl) adenine, molar yield 85.5%, HPLC purity 98.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com