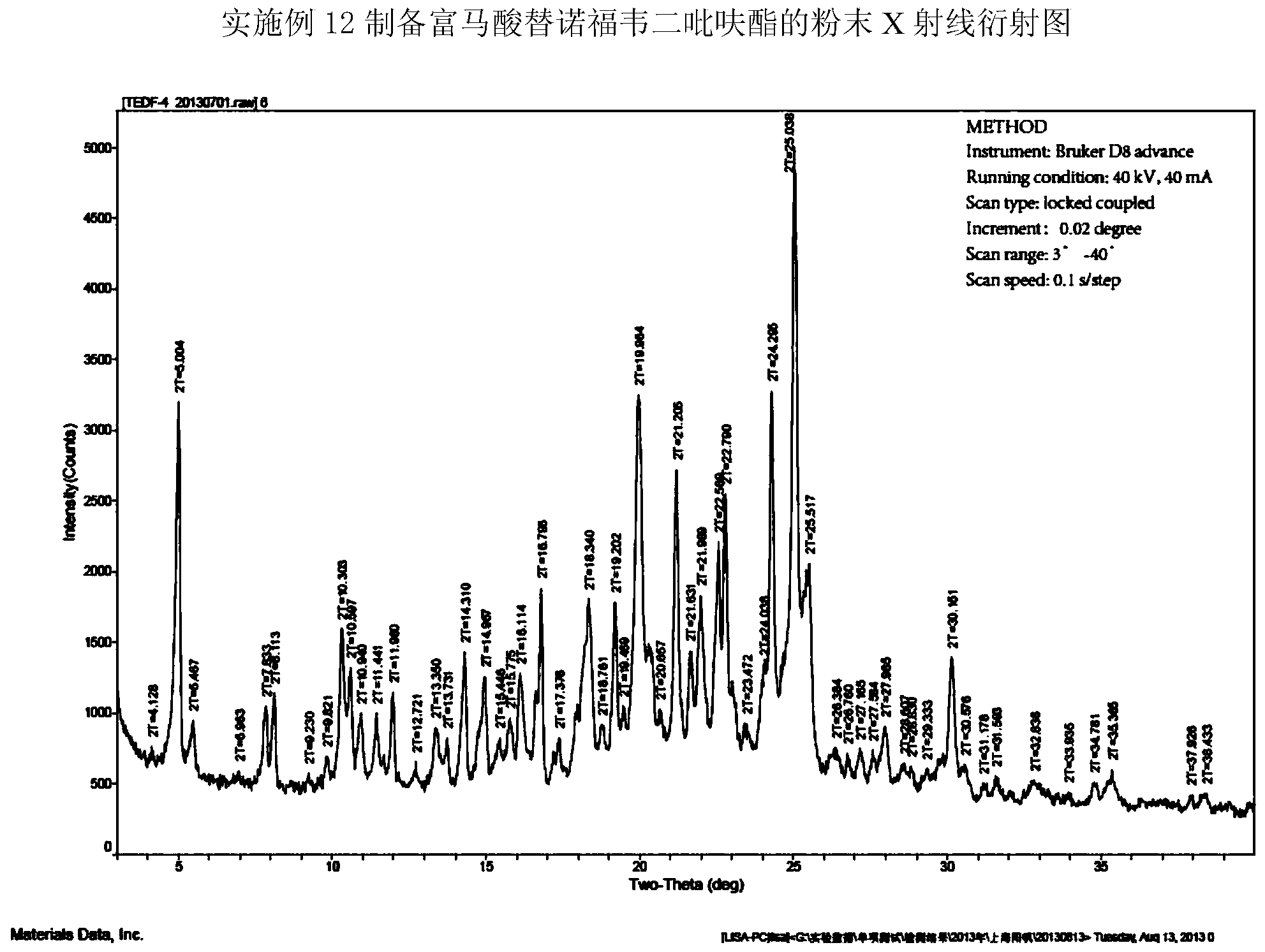
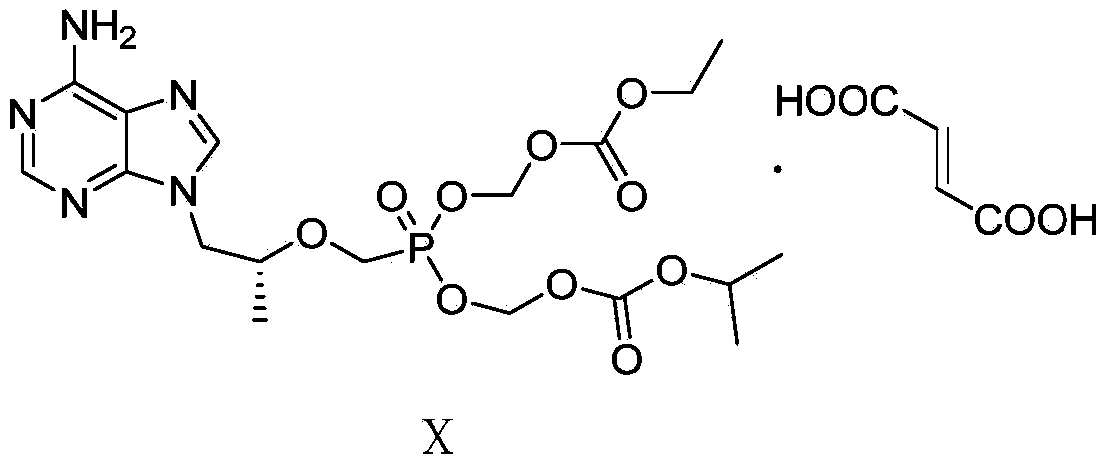
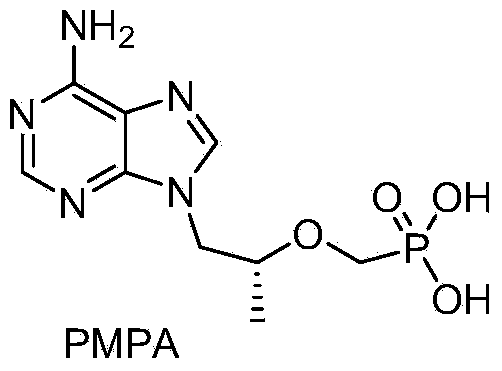
Tenofovir disoproxil fumarate and preparation method thereof
A technology of tenofovir fumarate and dipivoxil, which is applied in the field of medicine, can solve the problems of high process cost, high price, and easy explosion, and achieve the avoidance of genotoxic impurities, the production process is simple and easy to operate, and the production cost is reduced Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of embodiment 1 compound IX
[0048] Add 400g (1.24mol) of p-toluenesulfonyloxymethyl diethyl phosphate (formula VIII) and 100ml of N,N-dimethylformamide into the three-necked flask, stir for several minutes, then add 1250g of hydrobromic acid solution ( 40wt%) (6.20mol), N 2 Under gas protection, heat up to 95-100°C, react for 5 hours, cool down to room temperature, add 100ml of dichloromethane, stir, separate layers, take the water layer, adjust the pH value of the solution to 3.5 with cold saturated sodium hydroxide solution, and store at room temperature Stir for 0.5 hour, continue cooling to 0-5°C, and filter with suction to obtain 214 g of off-white solid with a yield of 65%.
Embodiment 2
[0049] The preparation of embodiment 2 compound IX
[0050] Add 400g (1.24mol) of p-toluenesulfonyloxymethyl diethyl phosphate (formula VIII) and 100ml of N,N-dimethylformamide into the three-necked flask, stir for several minutes, then add 2092g of hydrobromic acid solution ( 48wt%) (12.4mol), N 2 Under gas protection, heat up to 80-85°C, react for 5 hours, cool down to room temperature, add 100ml of dichloromethane, stir, separate layers, take the water layer, adjust the pH value of the solution to 3 with cold saturated sodium hydroxide solution, and keep at room temperature Stir for 0.5 hour, continue to cool to 0-5°C, and filter with suction to obtain 218 g of off-white solid with a yield of 66%.
Embodiment 3
[0051] The preparation of embodiment 3 compound IX
[0052] Add 400g (1.24mol) of p-toluenesulfonyloxymethyl diethyl phosphate (Formula VIII) and 100ml of N,N-dimethylformamide into the three-necked flask, stir for several minutes, then add 759g of bromotrimethylsilane (4.96mol), N 2 Under gas protection, heat up to 60-65°C, react for 5.5 hours, cool down to room temperature, add 120ml of ethyl acetate, stir, separate layers, take the water layer, adjust the pH value of the solution to 3.3 with cold saturated sodium hydroxide solution, and store at room temperature Stir for 0.5 hour, continue to cool to 0-5°C, and filter with suction to obtain 205 g of off-white solid with a yield of 62%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com