Adenine derivative and inclusion compound thereof
A technique of inclusion compound of adenine and cyclodextrin, which is applied in the direction of drug combination, non-active ingredients of polymer compounds, compounds of Group 5/15 elements of the periodic table, etc., which can solve the problem that the bioavailability is affected by food, etc. Achieve excellent bioavailability, good safety, and excellent antiviral activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
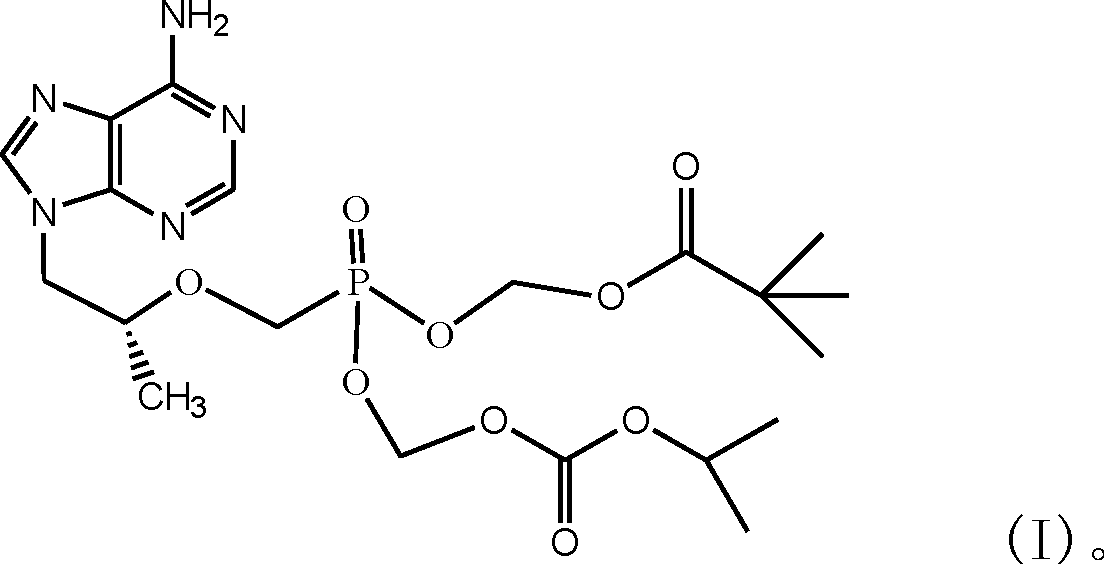
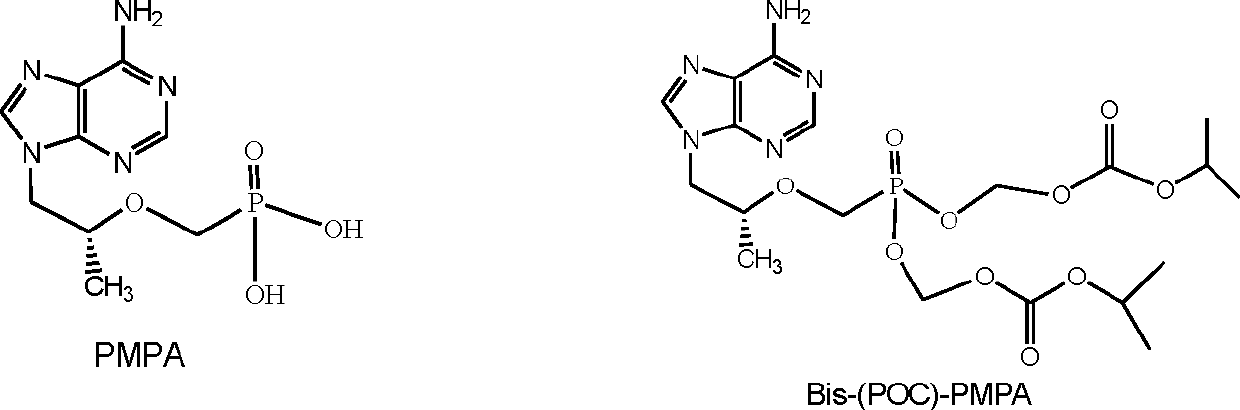
[0019] Example 1, Preparation of (R)-9-[2-(pivaloyloxymethyl)-(isopropoxycarbonyloxymethyl)phosphonomethoxypropyl]adenine (I)
[0020] 1.1. Synthesis of diethyl p-toluenesulfonyloxymethyl phosphate:
[0021] In a 1000ml three-necked flask, add 200ml toluene, 400ml diethyl phosphite, 120g paraformaldehyde and 50ml triethylamine, stir and heat to 70°C, keep the temperature for 2 hours, then heat up to reflux and continue the reaction until the TLC ( The developing agent uses n-hexane:ethyl acetate=1:4) and the reaction ends when diethyl phosphite cannot be detected. The solution was cooled to below 10°C, 560g of p-toluenesulfonyl chloride was added, and then 560ml of triethylamine was slowly added at about 5°C, maintaining the temperature not exceeding 10°C. After the dropwise addition, it was raised to room temperature and reacted for 8 hours until p-toluenesulfonyl chloride could not be detected by TLC. The solid was removed by suction filtration, and the filter cake was was...
Embodiment 2
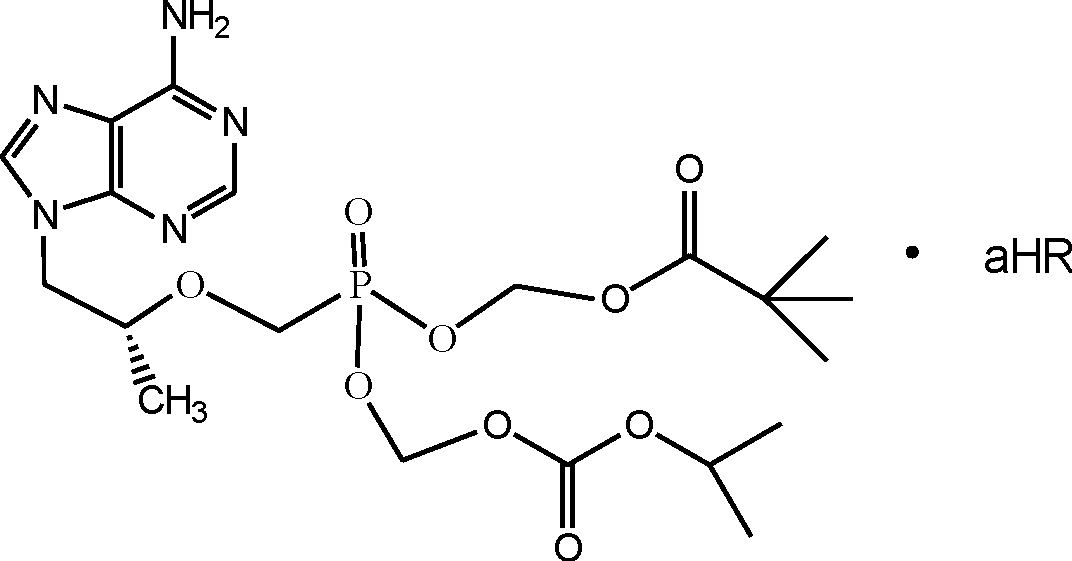
[0033] Example 2, β-cyclodextrin inclusion of (R)-9-[2-(pivaloyloxymethyl)-(isopropoxycarbonyloxymethyl)phosphonomethoxypropyl]adenine compound preparation
[0034] Weigh 20g of (R)-9-[2-(pivaloyloxymethyl)-(isopropoxycarbonyloxymethyl)phosphonomethoxypropyl]adenine, add 40ml of absolute ethanol to dissolve. Mix 45g of β-cyclodextrin with 567ml of water to make a 60°C saturated aqueous solution. Drop the ethanol solution of (R)-9-[2-(pivaloyloxymethyl)-(isopropoxycarbonyloxymethyl)phosphonomethoxypropyl]adenine into β-cyclodextrin In a saturated aqueous solution, keep stirring for 30 minutes, stop heating and continue stirring for 4 hours. Put it in the refrigerator to freeze for 24 hours, filter it with suction, wash the filter cake with absolute ethanol, dry it under reduced pressure, and grind it finely to get (R)-9-[2-(pivaloyloxymethyl)-(isopropoxy Carbonyloxymethyl)phosphonomethoxypropyl]adenine β-cyclodextrin inclusion complex 62.5g.
Embodiment 3
[0035] Example 3, Preparation of (R)-9-[2-(pivaloyloxymethyl)-(isopropoxycarbonyloxymethyl)phosphonomethoxypropyl]adenine fumarate
[0036] Take 5.3g of (R)-9-[2-(pivaloyloxymethyl)-(isopropoxycarbonyloxymethyl)phosphonomethoxypropyl]adenine dissolved in 30ml of ethanol, stir, slowly Add 10ml of ethanol solution containing 1.16g of fumaric acid dropwise, and finish the dropwise addition in about 30 minutes, continue stirring for 1 hour, and filter with suction, stir the filtrate to cool down to 0-4°C, continue stirring for 5 hours, and filter with suction to obtain 4.8 grams of white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com