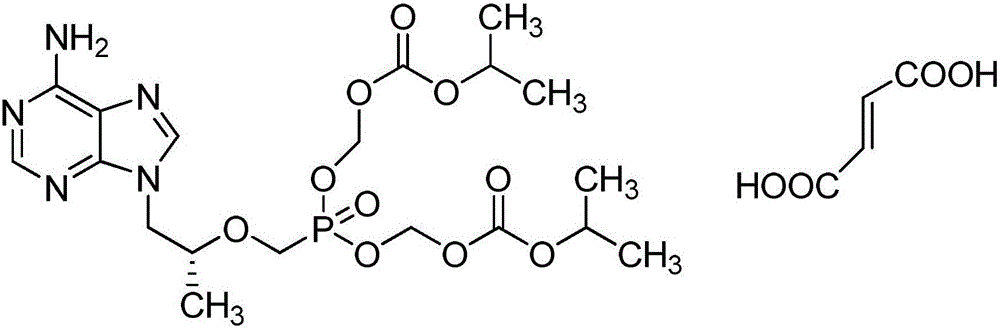
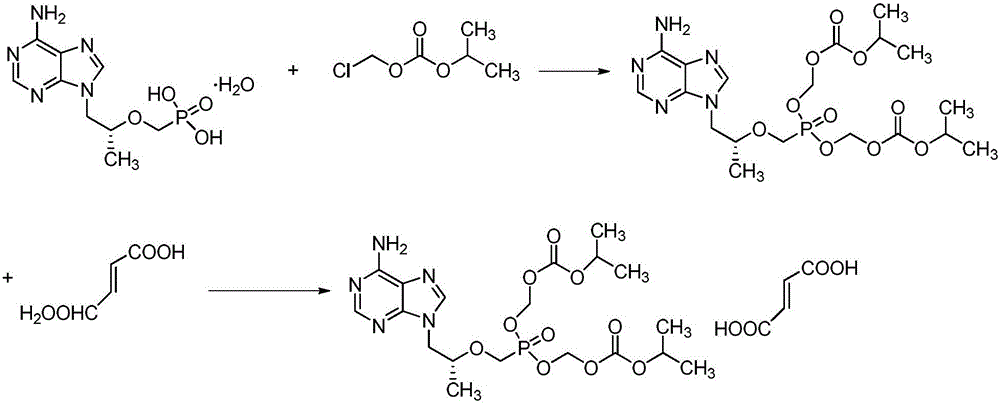
Preparation methods of tenofovir disoproxil and fumarate thereof
A technology of tenofovir and dipivoxil, which is applied in the field of compound preparation, can solve problems such as high solubility, low yield of salt-forming reaction, and excessive monoester content, and achieve simple post-processing, low impurity content, and high yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
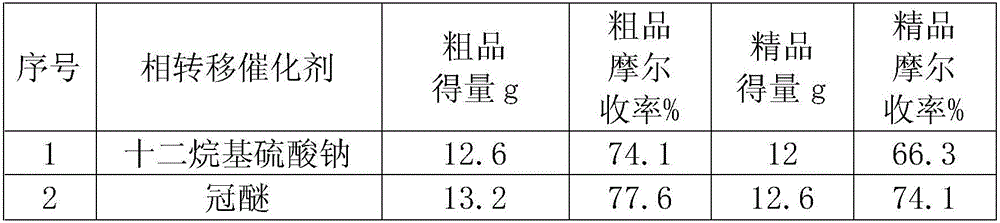
[0019] The impact of using different phase transfer catalysts on the reaction yield of tenofovir disoproxil:
[0020] In the reaction flask, add tenofovir monohydrate 10g (0.0328mol), phase transfer catalyst 0.025mol (see Table 1 for the selection of the phase transfer catalyst), 20mlN-methylpyrrolidone and 8.86g (0.0876mol) Triethylamine, warmed up to 50°C, stirred at this temperature for 0.5h. 21.4 g (0.14 mol) of chloromethyl isopropyl carbonate was slowly added dropwise to the reaction solution. After the dropwise addition was completed, the temperature was raised to 60° C. and stirred at this temperature for 4 h. After the reaction was completed, the reaction solution was poured into a cooled supersaturated aqueous sodium chloride solution, and stirred at -10°C for 24 hours. Suction filtration, washing with cold water, and the obtained solid was dried at 25°C in a blast drying oven. The yield and yield of the crude product are shown in Table 1. The crude product was adde...
Embodiment 2
[0025] The impact of adopting different highly polar organic solvents on the reaction yield of tenofovir disoproxil:
[0026]Add tenofovir monohydrate 10g (0.0328mol), tetrabutylammonium bromide 8g (0.0248mol), 20ml highly polar organic solvent (see the table for the selection of the highly polar organic solvent) in the reaction flask successively 2) and 8.86g (0.0876mol) of triethylamine, heated up to 50°C, and stirred at this temperature for 0.5h. 21.4 g (0.14 mol) of chloromethyl isopropyl carbonate was slowly added dropwise to the reaction solution. After the dropwise addition was completed, the temperature was raised to 60° C. and stirred at this temperature for 4 h. After the reaction was completed, the reaction solution was poured into a cooled supersaturated aqueous sodium chloride solution, and stirred at -10°C for 24 hours. Suction filtration, washing with cold water, the obtained solid was dried at 25°C in a blast drying oven, the yield and yield of the crude produ...
Embodiment 3
[0030] The impact of adopting different acid-binding agents on the reaction yield of tenofovir disoproxil:
[0031] Add tenofovir monohydrate 10g (0.0328mol), tetrabutylammonium bromide 8g (0.0248mol), 20mlN-methylpyrrolidone and 0.088mol acid-binding agent successively in reaction flask (the selection of described acid-binding agent The situation is shown in Table 3), the temperature was raised to 50°C, and stirred at this temperature for 0.5h. 21.4 g (0.14 mol) of chloromethyl isopropyl carbonate was slowly added dropwise to the reaction solution. After the dropwise addition was completed, the temperature was raised to 60° C. and stirred at this temperature for 4 h. After the reaction was completed, the reaction solution was poured into a cooled supersaturated aqueous sodium chloride solution, and stirred at -10°C for 24 hours. Suction filtration, washing with cold water, and the obtained solid was dried at 25°C in a blast drying oven. See Table 1 for the yield and yield of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com