Method for preparing donepezil hydrochloride crystal form I
A technology for donepezil hydrochloride and nepazil hydrochloride, applied in the field of preparing donepezil hydrochloride crystal form I, can solve the problems of increasing drying temperature, prolonging drying time, unable to reduce the minimum requirement of residual solvent, etc., to ensure stability and suitable for industrialization Production, the effect of shortening the drying time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: Preparation of the wet product of nepezil hydrochloride crystal form I
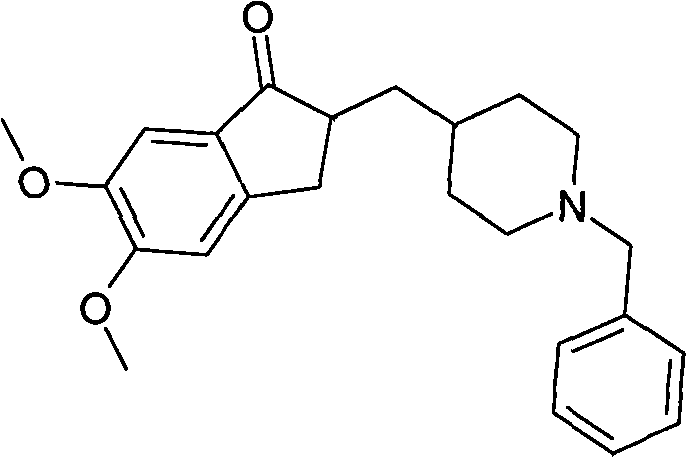
[0026] Add 20kg of donepezil base and 80L of methanol into the reaction kettle, heat to 40°C and stir to dissolve. Cool to 0-20°C, add 5.8kg concentrated hydrochloric acid / 20L methanol, control the temperature at 0-20°C, add 200L isopropyl ether after addition, stir at 0-20°C for 10-20min, filter to obtain hydrochloric acid Donepezil crystal form I wet product 40kg.
Embodiment 2
[0027] Embodiment 2: prepare the wet product of nepezil hydrochloride crystal form I
[0028] Add 20kg of donepezil hydrochloride, 80L of methanol, and 2kg of water into the reaction kettle, then heat to 40°C and stir to dissolve, cool to 0-20°C, add 200L of isopropyl ether, after the addition is complete, stir at 0-20°C for 10-20min, filter, Obtain 40kg of donepezil hydrochloride crystal form I wet product.
Embodiment 3
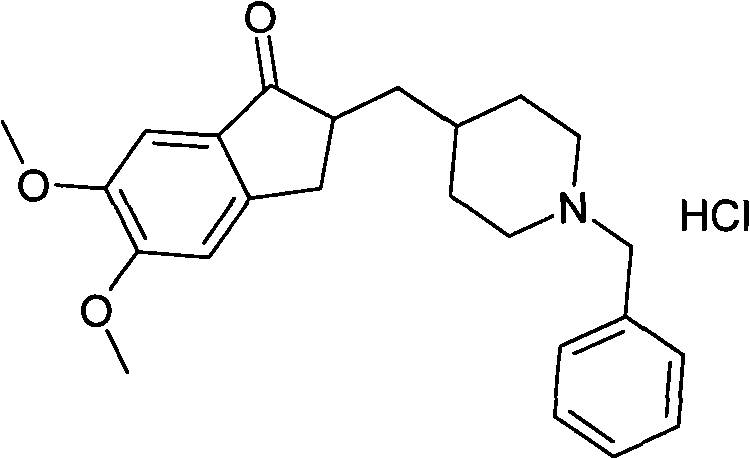
[0029] Example 3: Preparation of Nepezil Hydrochloride Form I Finished Product
[0030] The wet product of donepezil hydrochloride form I obtained in Example 2 was vacuum-dried at 40°C, 50°C and stepwise temperature rise conditions, and the vacuum degree was controlled within the range of -0.07Mpa to -0.1Mpa, and measured after a certain period of time The residual amount of the solvent of isopropyl ether and methyl alcohol, result is shown in table I, table II, table III respectively:
[0031] Table I (40°C)
[0032]
[0033] Table II (50°C)
[0034]
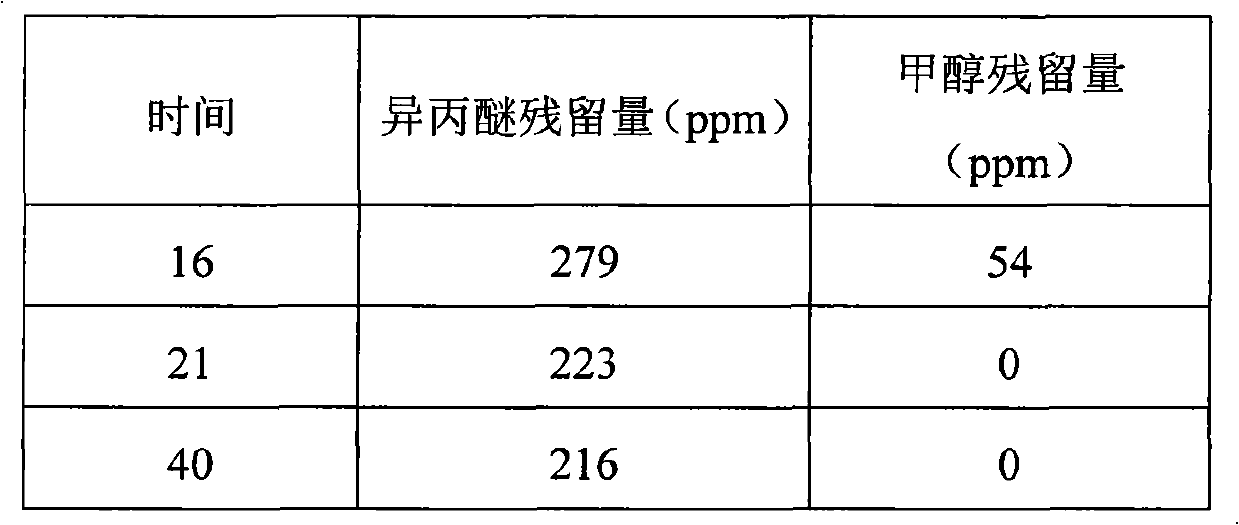
[0035] Table III (stepwise temperature rise)
[0036] time
[0037] 16
[0038] Judging from the data results in Table I and Table II, directly increase the drying temperature to 40°C and 50°C, and keep the temperature constant. After drying for 16 hours, the residual amount of isopropyl ether remains basically constant. The residues did not change much. The results in Table III show that by stepw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com