Synthesizing technology of donepezil hydrochloride
A technology of donepezil hydrochloride and synthesis process, applied in directions such as organic chemistry, can solve problems such as low total yield and many steps, and achieve the effects of high yield and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
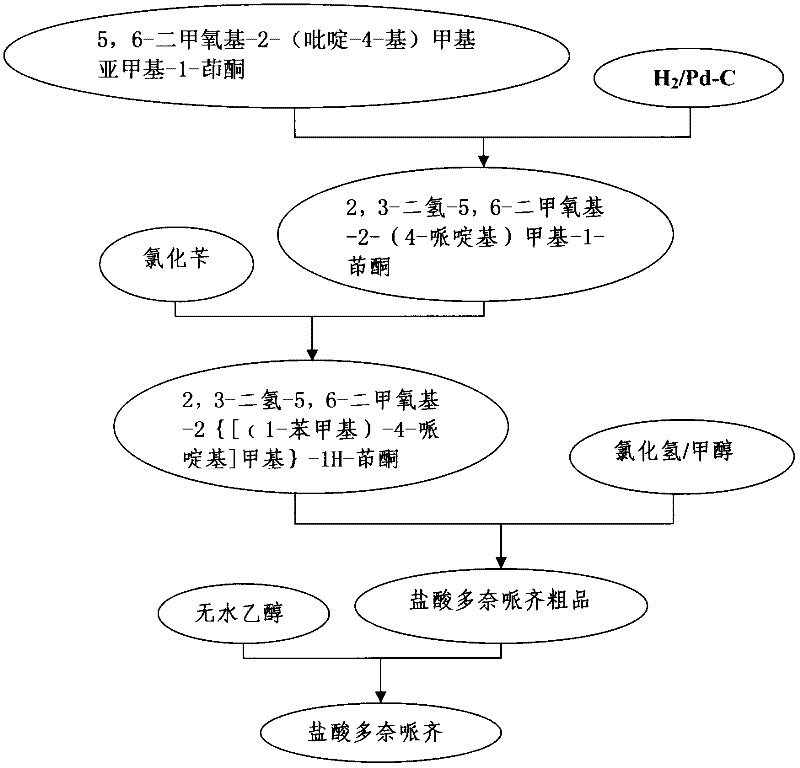
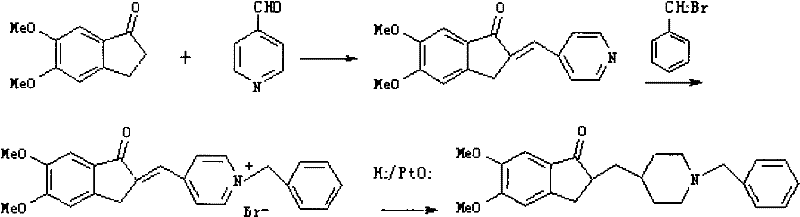
[0030] Preparation of 2,3-dihydro-5,6-dimethoxy-2-(4-piperidinyl)methyl-1-indanone (II):
[0031] In a 1L hydrogenation reactor, add 10g (0.035mol) of substituted indanone, 300ml of glacial acetic acid, 1g of 10% palladium charcoal, feed hydrogen at 75°C, pressurize at 0.35MPa for 1 hour, cool to 30°C, and filter out Palladium charcoal to obtain a hydrogenation solution, evaporate the solvent to glacial acetic acid at -0.08MPa and 95°C, add 10% sodium bicarbonate aqueous solution to neutralize the reaction product until the pH value is 7, add 200ml dichloromethane to extract three times, and the organic layer is extracted with 20g sodium bicarbonate Dry over sodium sulfate for 24 hours, remove sodium sulfate, and concentrate the filtrate to obtain 8.5 g of yellow oil (II), with a yield of 84%. The feed ratio (molar ratio) of the above reaction is: 5,6-dimethoxy-2-(pyridin-4-yl)methylmethylene-1-indanone:hydrogen=1:(3~5), Here the ratio hydrogen=(3~5) refers to any value betwe...
Embodiment 2
[0033] Preparation of 2,3-dihydro-5,6-dimethoxy-2-(4-piperidinyl)methyl-1-indanone (II):
[0034] In a 1L hydrogenation reactor, add 10g (0.035mol) of substituted indanone, 300ml of glacial acetic acid, 1g of 10% palladium on carbon, pass hydrogen gas at 75°C, pressurize at 0.35MPa for 2 hours, cool to 30°C, and filter out Palladium charcoal to obtain a hydrogenation solution, evaporate the solvent to glacial acetic acid at -0.08MPa and 95°C, add 10% sodium bicarbonate aqueous solution to neutralize the reaction product until the pH value is 7, add 200ml dichloromethane to extract four times, and use 20g After drying over anhydrous sodium sulfate for 20 hours, the sodium sulfate was removed, and the filtrate was concentrated to obtain 7.8 g of yellow oil (II), with a yield of 77%. The feed ratio (molar ratio) of the above reaction is: 5,6-dimethoxy-2-(pyridin-4-yl)methylmethylene-1-indanone:hydrogen=1:(3~5), The ratio hydrogen=(3~5) here refers to any value between 3~5, such ...
Embodiment 3
[0036] The preparation of donepezil hydrochloride (I):
[0037] Add 30g (0.104mol) hydride and 500ml dichloromethane into a dry 1L three-necked flask, stir, dissolve at 35°C, add dropwise 45ml (0.325mol) of triethylamine, continue stirring for 10 minutes, and add dropwise 40ml ( 0.345 mol) of benzyl chloride, maintained at 35°C and continued to react for 4 hours, cooled to 20-25°C, filtered with suction, discarded the filter residue, concentrated the filtrate, dissolved the concentrate in 300ml of methanol, added dropwise 10% methanolic hydrogen chloride solution to form a salt, Cool and crystallize at 4°C, filter, wash the filter cake with 50ml of methanol, and dry at 58-60°C to obtain 24g of crude donepezil hydrochloride. After filtration, the solid was dried at 58-60° C. to obtain 15 g of donepezil hydrochloride (I), with a yield of 61%. The feed ratio (molar ratio) of the above reaction is: compound II: benzyl chloride: 10% methanolic hydrogen chloride solution=1: (3~5): ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com