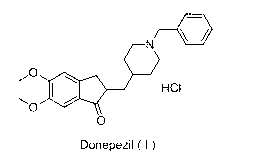
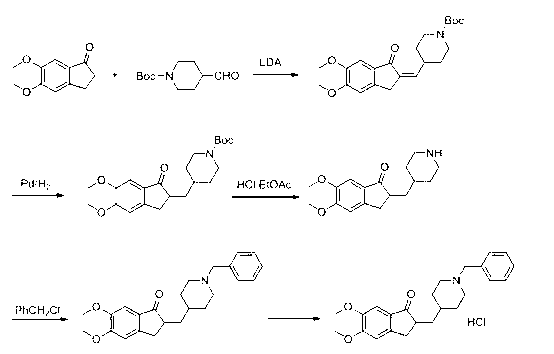
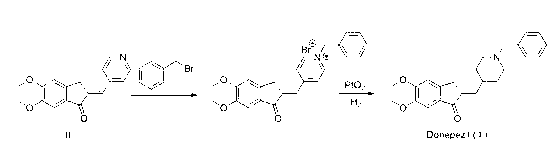
Method for preparing donepezil hydrochloride
A technology of donepezil hydrochloride and organic acid, which is applied in the field of preparation of donepezil hydrochloride, can solve problems such as poor reduction stability, serious environmental pollution, environmental and worker hazards, and achieve the effects of mild reaction conditions, simple and efficient operation, and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of Donepezil Hydrochloride
[0039] Prepare donepezil hydrochloride, comprising the following steps:
[0040] 1) Preparation of compound 5,6-dimethoxy-2-(4-piperidinyl)methylene-2,3-dihydro-1-indanone (Ⅲ)
[0041] Put 150g (0.53mol) of 5,6-dimethoxy-2-(4-pyridylmethylene)-1-indanone, 10g (0.044mol) of platinum dioxide, 2000ml of methanol, and 200ml of acetic acid into high-pressure hydrogenation In the still, use the decompression replacement method to drive out the air in the still, and pressurize (7-7.5kg) at 40°C-45°C to hydrogenate until no hydrogen is absorbed (more than 40 hours). Filter, and recover the solvent under reduced pressure at 45°C-75°C. Add an appropriate amount of water to dissolve the product, and adjust the pH to 11-12 in an ice bath. Extract with dichloromethane, combine the organic layers, and wash with saturated brine until the pH is between 8-9. Stir and dry with anhydrous sodium sulfate, and recover the solvent ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com