Donepezil hydrochloride dispersible tablet and preparation method thereof
A technology of donepezil hydrochloride and dispersible tablets, applied in the directions of heterocyclic compound active ingredients, pill delivery, nervous system diseases, etc., can solve problems such as unfavorable drug dissolution and absorption, achieve stable indicators, be beneficial to dissolution and absorption, and prolong storage period Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
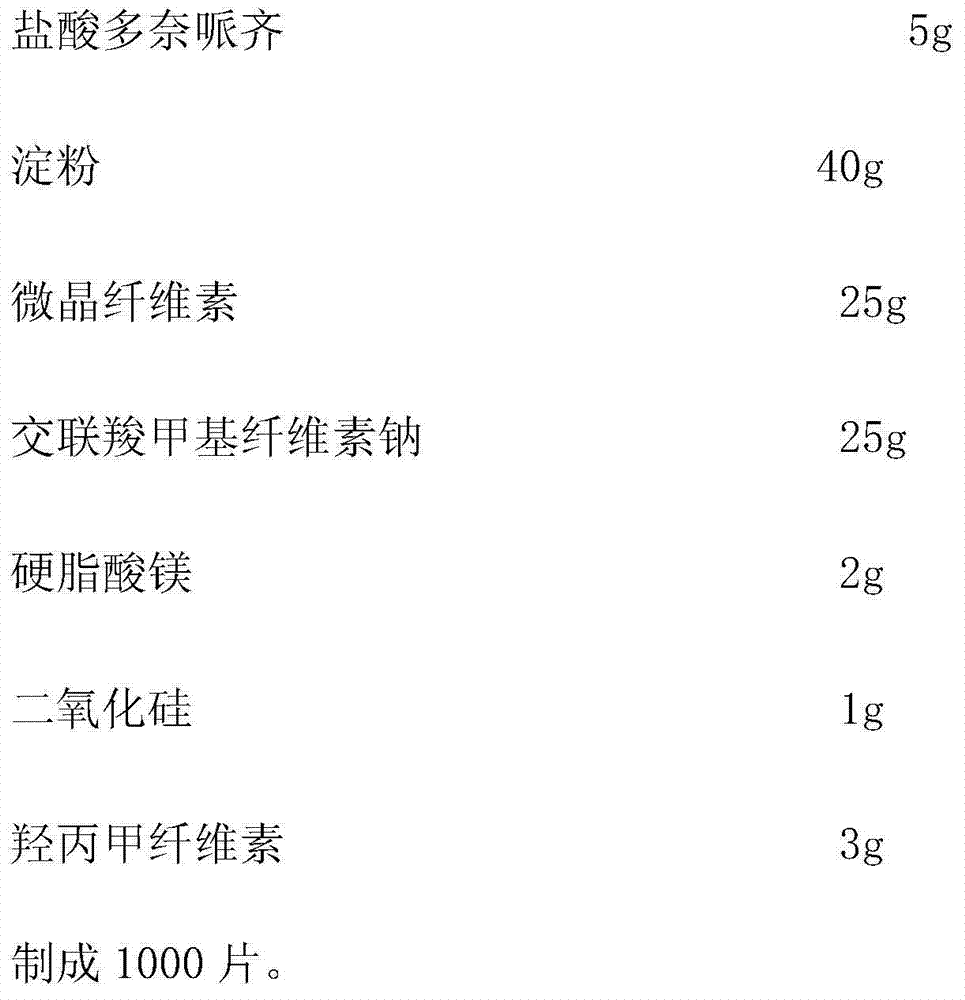
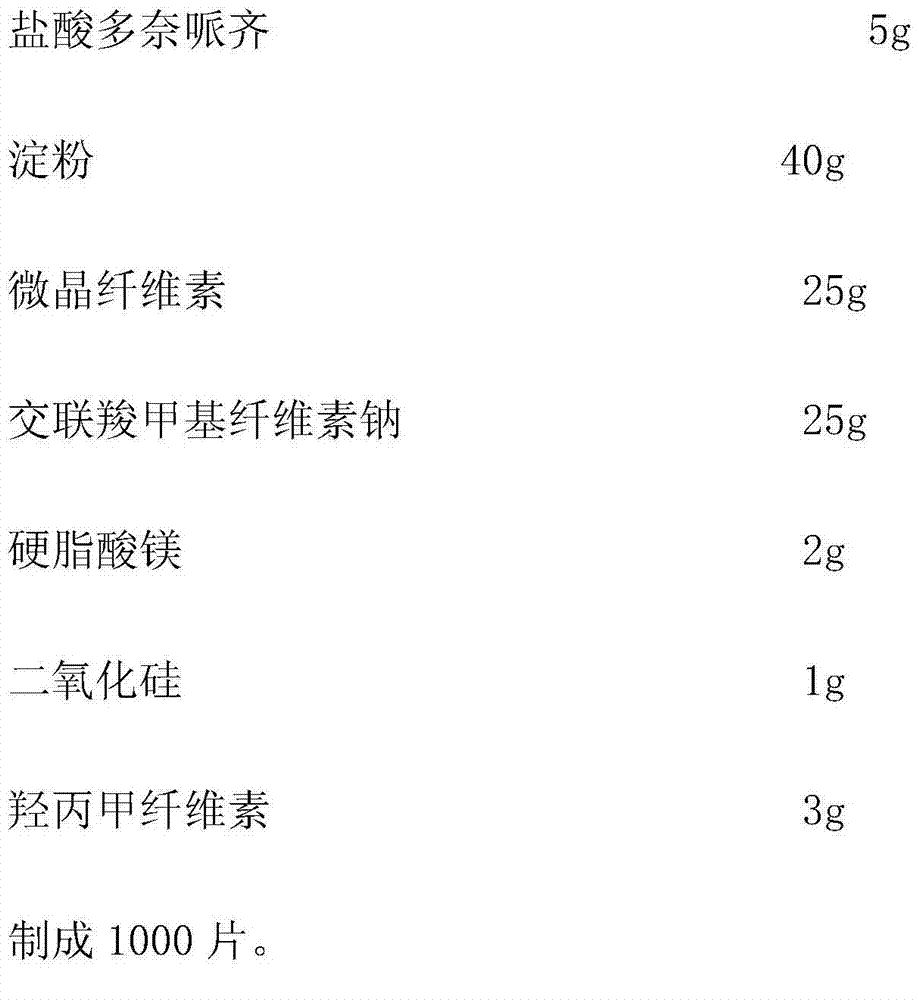
[0037] Embodiment 1: Donepezil hydrochloride dispersible tablet, the components are as follows:
[0038]
[0039] The preparation method is as follows:
[0040] (1) First pass the donepezil hydrochloride, starch, and microcrystalline cellulose through a 100-mesh sieve for later use.
[0041](2) Weigh the starch and microcrystalline cellulose according to the prescription, and mix them evenly. Add the prescribed amount of donepezil hydrochloride, mix well, add binder 1% hypromellose aqueous solution to make soft material, granulate with 20 mesh sieve, dry at 50-60°C, granulate with 20 mesh sieve, add cross-linked carboxyl Sodium methylcellulose, magnesium stearate, and silicon dioxide are mixed evenly.
[0042] (3) Take a sample to test the particle content, calculate the tablet weight according to the measured particle content, and compress the tablet.
[0043] (4) Sampling and full inspection, and packaging to obtain finished products after passing the test.
[0044] T...
Embodiment 2
[0045] Embodiment 2: Donepezil hydrochloride dispersible tablets, the components are as follows:
[0046]
[0047] The preparation method is as follows:
[0048] (1) First pass the donepezil hydrochloride, starch, and microcrystalline cellulose through a 100-mesh sieve for later use.
[0049] (2) Weigh the starch and microcrystalline cellulose according to the prescription, and mix them evenly. Add the prescribed amount of donepezil hydrochloride, mix well, add the soft material made of polyvinylpyrrolidone as the binder, granulate with a 20-mesh sieve, dry at 50-60°C, granulate with a 20-mesh sieve, add croscarmellose sodium , magnesium stearate, and micropowder silica gel.
[0050] (3) Take a sample to test the particle content, calculate the tablet weight according to the measured particle content, and compress the tablet.
[0051] (4) Sampling and full inspection, and packaging to obtain finished products after passing the test.
[0052] It completely disintegrates ...
Embodiment 3
[0053] Embodiment 3: Donepezil hydrochloride dispersible tablets, the components are as follows:
[0054]
[0055] The preparation method is as follows:
[0056] (1) Pass the donepezil hydrochloride and the starch through a 100-mesh sieve respectively, and set aside.
[0057] (2) Weigh the starch according to the prescription, add the prescribed amount of donepezil hydrochloride, mix evenly, add 5% starch slurry as binder to make soft material, granulate with 20 mesh sieve, dry at 50-60°C, and granulate with 20 mesh sieve , add crospovidone and magnesium stearate and mix well.
[0058] (3) Take a sample to test the particle content, calculate the tablet weight according to the measured particle content, and compress the tablet.
[0059] (4) Sampling and full inspection, and packaging to obtain finished products after passing the test.
[0060] The disintegration time limit is completely disintegrated in 37°C water within 2 minutes and passes through the No. 2 sieve, whic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com