Preparation method of donepezil hydrochloride impurities
A technology for donepezil hydrochloride and impurities, applied in the field of preparation of donepezil hydrochloride impurities, can solve problems such as high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
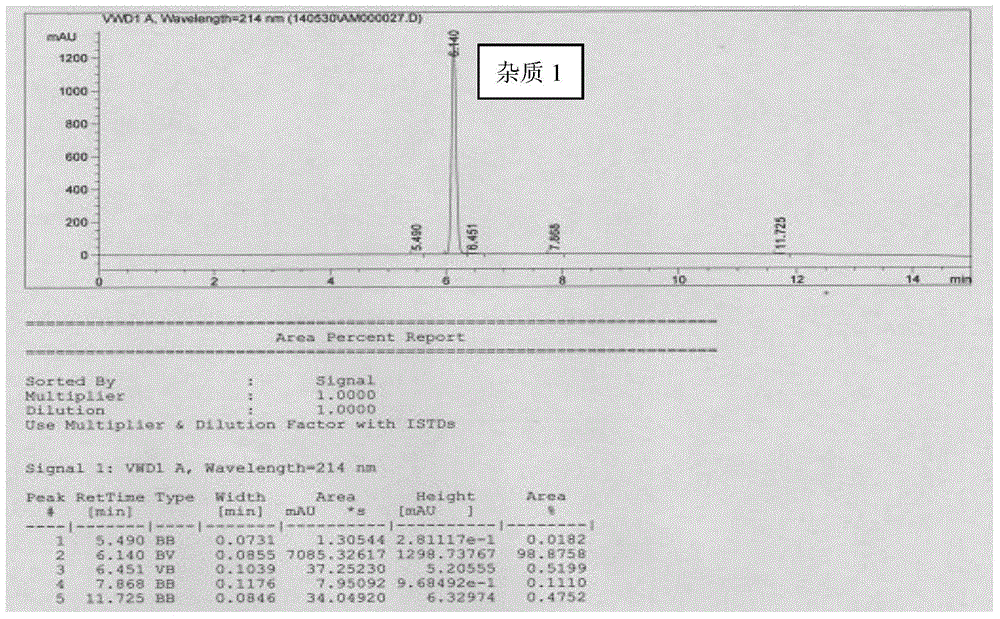
[0024] Example 1 Preparation of donepezil hydrochloride impurity 1
[0025] The preparation of donepezil hydrochloride impurity 1 comprises the following steps:
[0026] Add 5,6-dimethoxy-2-(4-piperidinylmethyl)-1-indanone (SM, 20g, 61.5mmol) into 200ml water at room temperature, cool down to 0°C, and divide at 0°C Sodium borohydride (20g, 528mmol) was added three times. After the addition was complete, it was stirred at 0°C for 0.5 hours, and the temperature was raised to 25°C-30°C within 1 hour, and kept stirring at 25-30°C for 1 hour. The system was adjusted to pH = 1 with concentrated hydrochloric acid, continued to stir at 25-30°C for 1 hour, adjusted to pH = 10 with 40% sodium hydroxide, extracted three times with ethyl acetate, combined the organic phase, washed the organic phase with saturated brine, and anhydrous sodium sulfate dry. Filtration and concentration gave a white solid (Compound I, 12.3 g, 73%).
[0027] Compound I (1.4g, 5.13mmol) was added to 15ml of m...
Embodiment 2
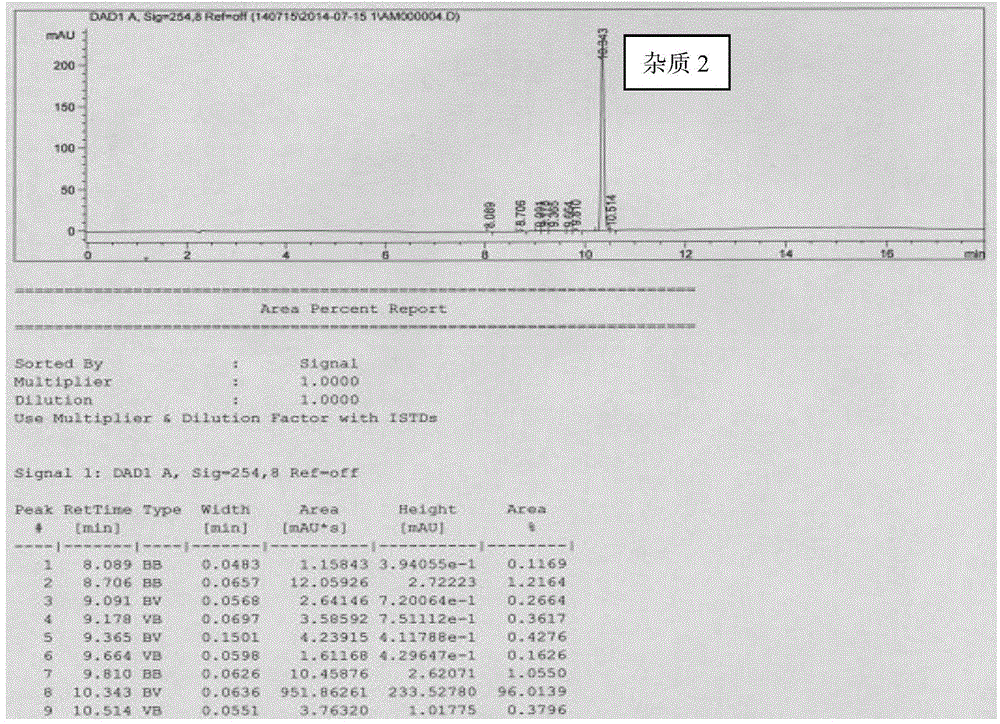
[0029] Example 2 Preparation of donepezil hydrochloride impurity 2
[0030] Preparation of donepezil hydrochloride impurity 2 comprises the following steps:
[0031] Compound I (800mg, 2.93mmol) was added to 5ml of formic acid at room temperature, heated to reflux with stirring, kept stirring for 2 hours, concentrated the reaction system, and the concentrate was purified by silica gel column (PE:EA volume ratio: 1:1), collected The eluent containing impurity 2 was concentrated to dryness to obtain a light red oil, which was solid at -20°C for 5 days (impurity 2, 370 mg, 41%). MS-ESI m / z:301.17[M+H] + 302.1. 1 H-NMR (400MHz, CDCl 3 )δ8.01(1H, s), 7.00(1H, s), 6.86(1H, s), 6.43(1H, s), 4.39(1H, d, J=13.2Hz), 3.89(3H, s), 3.88(3H,s), 3.60(1H,d,J=13.2Hz), 3.25(2H,s), 3.08~3.02(1H,m), 2.66~2.59(1H,m), 2.42(2H,d, J=6.4Hz), 1.81 (3H,t, J=13.2Hz), 1.19~1.08(2H,m). HPLC detection results such as figure 2 shown.
Embodiment 3
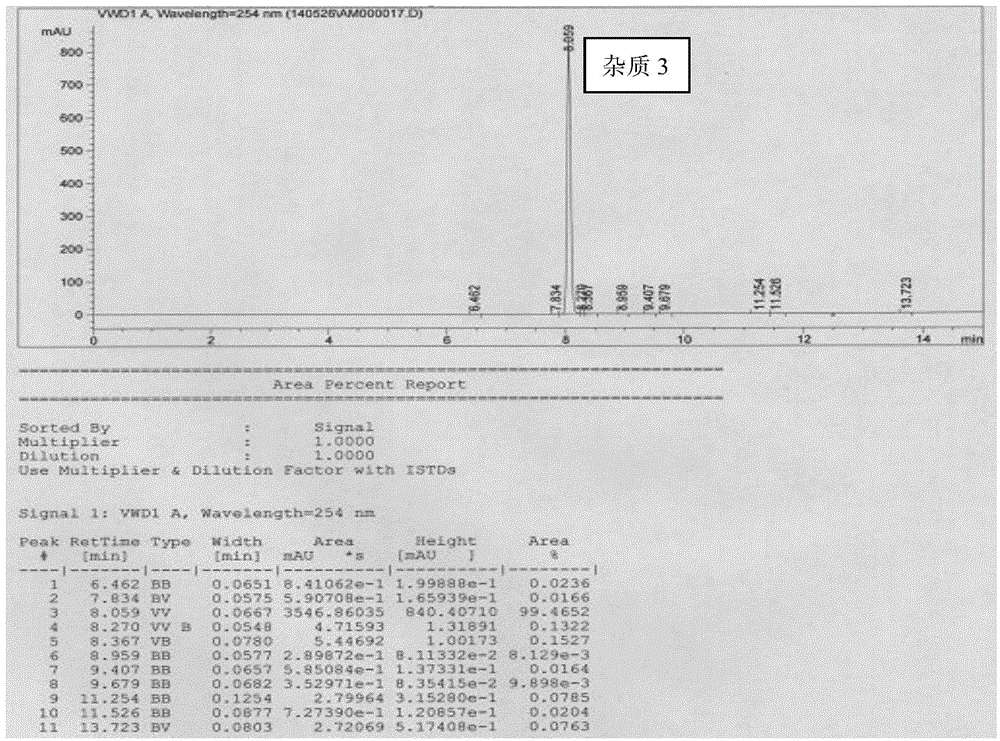
[0032] Example 3 Preparation of donepezil hydrochloride impurity 3
[0033] Preparation of donepezil hydrochloride impurity 3 comprises the following steps:
[0034] Add 15ml of methanol and 20ml of purified water to the reaction flask at room temperature, then add 5,6-dimethoxy-2-(4-piperidinylmethyl)-1-indanone (3.5g, 10.8mmol), carbonic acid Potassium (2.23 g, 16.2 mmol) was stirred at room temperature for 2 hours. Concentrate under reduced pressure to remove methanol, extract with ethyl acetate, separate the organic phase, dry over anhydrous sodium sulfate, filter and concentrate. Add 25ml of isopropyl formate to the concentrate, heat to 50°C, keep stirring for 2 hours, concentrate the reaction system under reduced pressure, purify the concentrate through a silica gel column (PE:EA volume ratio is 2:1), and collect the impurity 3 The eluent was concentrated to dryness to give a yellow solid (impurity 3, 2.9 g, 88%). MS-ESI m / z:317.16[M+H] + 318.1. 1 H-NMR (400MHz, CDC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com