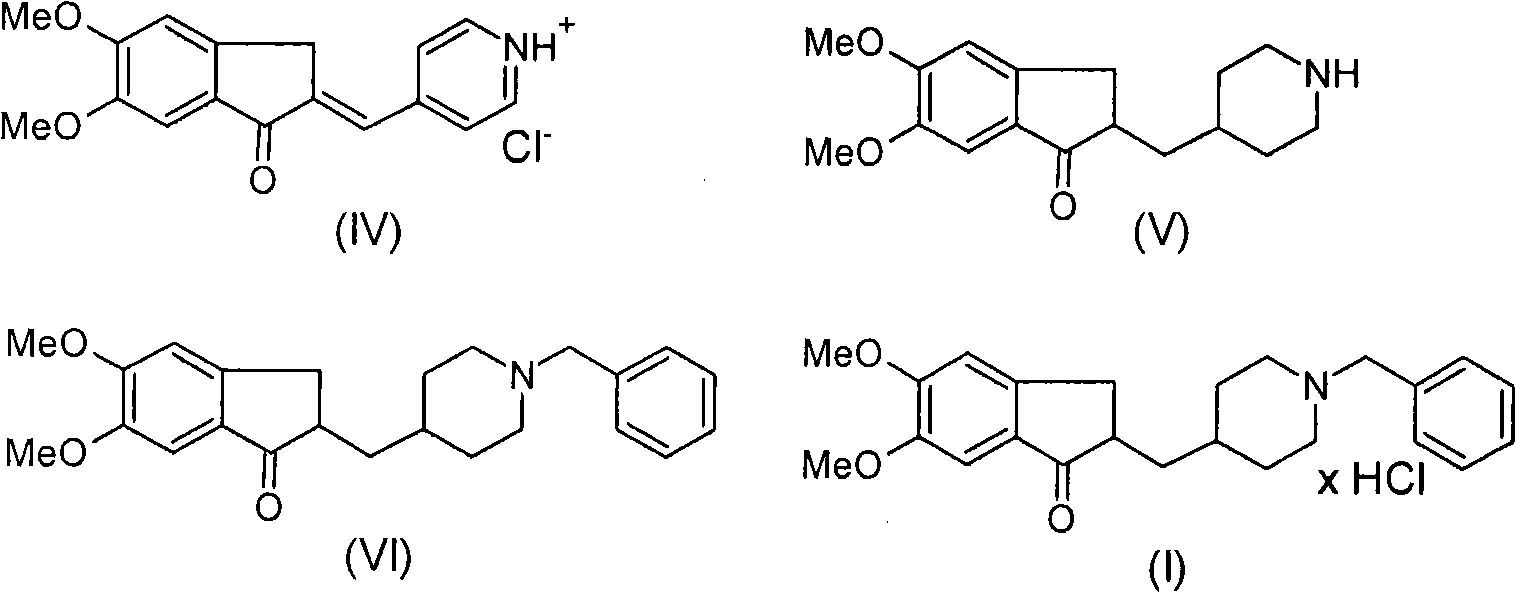
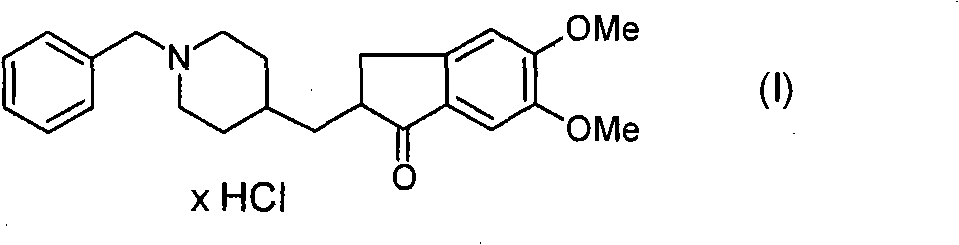
Novel process for production of highly pure polymorph (I) donepezil hydrochloride
A kind of technology of donepezil hydrochloride and donepezil base, applied in the field of 1-benzyl-4-[-2-yl]methylpiperidine hydrochloride, can solve the problem of expensive method, can not avoid the risk of insufficient hydrogenation and overhydrogenated products , can not completely control the hydrogenation and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: Preparation of 2-(4-piperidinylmethyl)-5,6-dimethoxy-1-indanone-p-toluenesulfonic acid according to Example 2 in PCT publication No.WO 2005 / 044805A1 Salt reproduction experiment:
[0057] 4.02 g of 2-(4-pyridyl (piridyl) methylene)-5,6-dimethoxy-1-indanone p-toluenesulfonate were dissolved in 300 ml of absolute methanol, followed by addition of 330 mg PtO 2 catalyst, and the mixture was hydrogenated for 10.5 hours at atmospheric pressure with stirring at room temperature. The solid was filtered off and washed with 50 mL of anhydrous methanol. The liquid phase was evaporated to dryness, the residue was dissolved in 150 ml of dry isopropanol while heating, and the solution was cooled to crystallize to obtain 2.02 g of the title compound. The mother liquor was evaporated to a volume of 15ml to give a further 0.46g of material. The two fractions were combined to obtain 2.86 g of the title compound.
[0058] The results of the HPLC analysis on the product con...
Embodiment 2
[0060] Embodiment 2: Preparation of 2-(4-piperidinylmethyl)-5,6-dimethoxy-1-indanone hydrochloride according to Example 2 in PCT publication No.WO 2005 / 044805A1 Reproduce experiment:
[0061] 3.17 g of 2-(4-pyridylmethylene)-5,6-dimethoxy-1-indanone hydrochloride was dissolved in 300 mL of anhydrous methanol, followed by the addition of 330 mg of PtO 2 catalyst, and the mixture was hydrogenated for 10.5 hours at atmospheric pressure with stirring at room temperature. The solid was filtered off and washed with 50 mL of anhydrous methanol. The liquid phase was evaporated to dryness, the residue was dissolved in 150 ml of anhydrous isopropanol while heating, and the solution was cooled to 0°C for crystallization to obtain 2.02 g of the title compound. The mother liquor was evaporated to a volume of 15ml to give a further 1.11g of material. The two fractions were combined to obtain 3.13 g of the title compound.
[0062] The results of the HPLC analysis on product content are s...
Embodiment 3
[0064] Example 3: 200 liters of acetic acid, 2.2 kg of charcoal containing 10% palladium suspended in 22 liters of acetic acid and 22.24 kg of 4-[(5,6-dimethoxy-1-indanone)-2- Subunit]-methyl-pyridine hydrochloride (IV) was measured into a 500 liter inert hydrogenation autoclave, and the mixture was hydrogenated at 68-72° C. under a 5-atm overpressure while vigorously stirring until the pressure dropped to until the end. The autoclave was cooled to 20-25°C and the catalyst was filtered off. The filtrate was concentrated in vacuo to a volume of 66 liters, to which 72 liters of methyl-isobutyl-ketone were then added dropwise with stirring. The crystalline material was filtered off and washed with methyl-isobutyl-ketone. The wet material was dissolved in 210 liters of boiling methanol, which was then cooled to 0-5°C. The crystalline material was filtered off, washed and dried to obtain 15.12 kg of 4-[(5,6-dimethoxy-1-indanon)-2-yl]-methyl-piperidine (V).
[0065] The results ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com