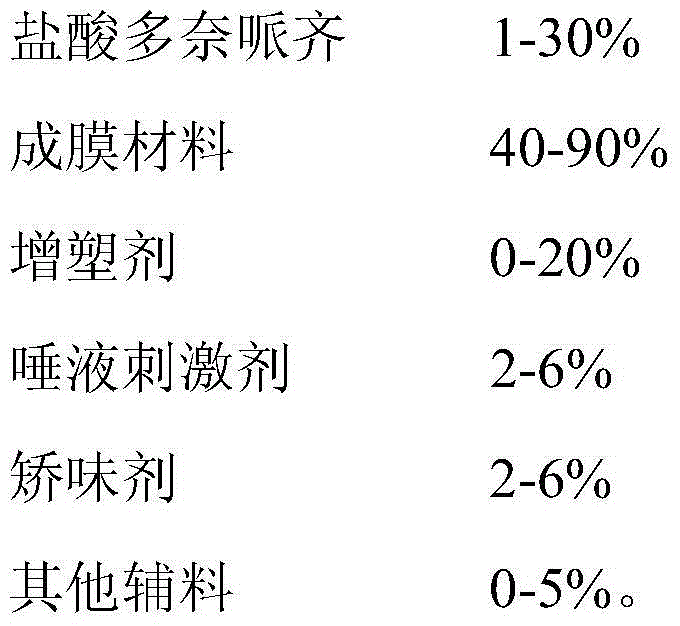
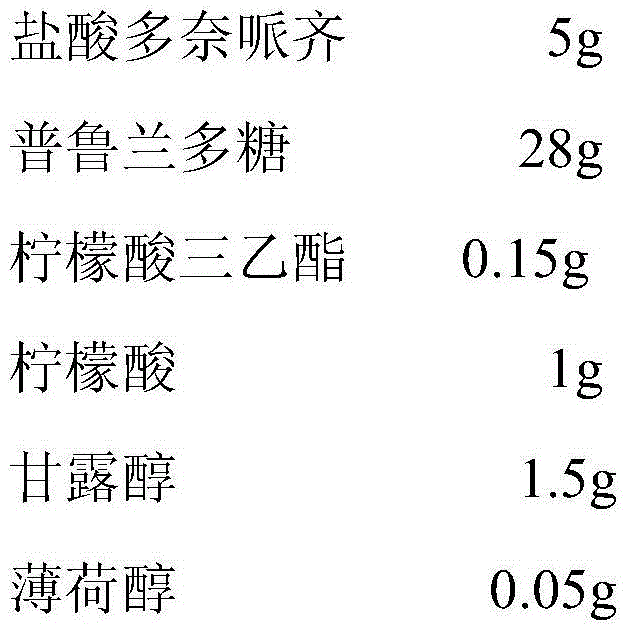
Preparation method of donepezil oral fast dissolving film
A technology of donepezil hydrochloride and oral fast-dissolving film agent, applied in the field of chemical pharmacy, can solve the problems of difficult control of conditions, complicated process and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015]
[0016]
[0017] Take the donepezil hydrochloride and pullulan of the prescribed amount, place them in an appropriate container, add 100ml of purified water, stir and dissolve at 50°C, add the prescribed amount of triethyl citrate, citric acid, mannitol, and menthol, and (10000rpm) under high-speed shearing for 3 minutes, repeat 3 times, and stand still under vacuum for 8 hours to remove air bubbles, and then the gel for scraping coating is obtained. Scrape-coat this glue solution on a stainless steel plate with a film coater, and dry it at a temperature between 50°C and 60°C. Then the film agent obtained by scraping is peeled off from the plastic film, cut and packaged to obtain the film agent.
Embodiment 2
[0019]
[0020] Take donepezil hydrochloride and polyvinyl alcohol 0588 of prescription quantity, be placed in appropriate container, add 100ml purified water, after stirring and dissolving at 50 ℃, add triethyl citrate, sodium citrate, mannitol of prescription quantity, at (10000rpm ) under high-speed shearing for 3 minutes, repeated 3 times, and left to stand under vacuum for 6 hours to remove air bubbles to obtain the gel for scraping. Scrape-coat this glue solution on a stainless steel plate with a film coater, and dry it at a temperature between 50°C and 60°C. Then the film agent obtained by scraping is peeled off from the plastic film, cut and packaged to obtain the film agent.
Embodiment 3
[0022]
[0023]
[0024] Weigh the prescribed amount of donepezil hydrochloride and polyoxyethylene N10, put them in an appropriate container, add 100ml of purified water, stir and dissolve at 50°C, add the prescribed amount of triethyl citrate, malic acid, mannitol, and menthol, and (10000rpm) under high-speed shearing for 3 minutes, repeat 3 times, and stand still under vacuum for 8 hours to remove air bubbles, and then the gel for scraping coating is obtained. Scrape-coat this glue solution on a stainless steel plate with a film coater, and dry it at a temperature between 40°C and 50°C. Then the film agent obtained by scraping is peeled off from the plastic film, cut and packaged to obtain the film agent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com