Donepezils compound long-acting slow-releasing and controlled-releasing composition and preparation method thereof
A technology of donepezil and compounds, which is applied in the field of pharmaceutical preparations to achieve the effects of reducing the number of times of taking medicines, maintaining stable product quality, and mature preparation technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Weigh 100 mg of carrier material acrylic resin and dissolve in 2 ml of acetone, drop into 5 ml of donepezil hydrochloride aqueous solution under cooling and magnetic stirring, and add 3 mg of magnesium stearate, slowly drop the mixture into 25 ml of liquid paraffin containing 0.5% Span60 to form The liquid paraffin wraps the acetone emulsifier, and gradually raises the temperature. After 2 to 3 hours, the microparticles are precipitated, washed with n-hexane for several times, and dried under reduced pressure to obtain the acrylic resin long-acting sustained and controlled release composition encapsulating donepezil hydrochloride.
Embodiment 2
[0043] The long-acting sustained and controlled-release composition of donepezil hydrochloride polylactic acid was prepared by emulsification-solvent evaporation method. 100 mg of carrier material polylactic acid was dissolved in 2 ml of chloroform, and 20 mg of donepezil hydrochloride was dispersed in it. Stirring at 800 rpm, slowly added to the dispersion After being emulsified in the medium (1% PVA solution) for 10 minutes, pour into the diffusion medium (0.2% PVA solution) to volatilize the organic solvent, then centrifuge, filter, and dry to obtain the donepezil hydrochloride long-acting sustained and controlled release composition.
Embodiment 3
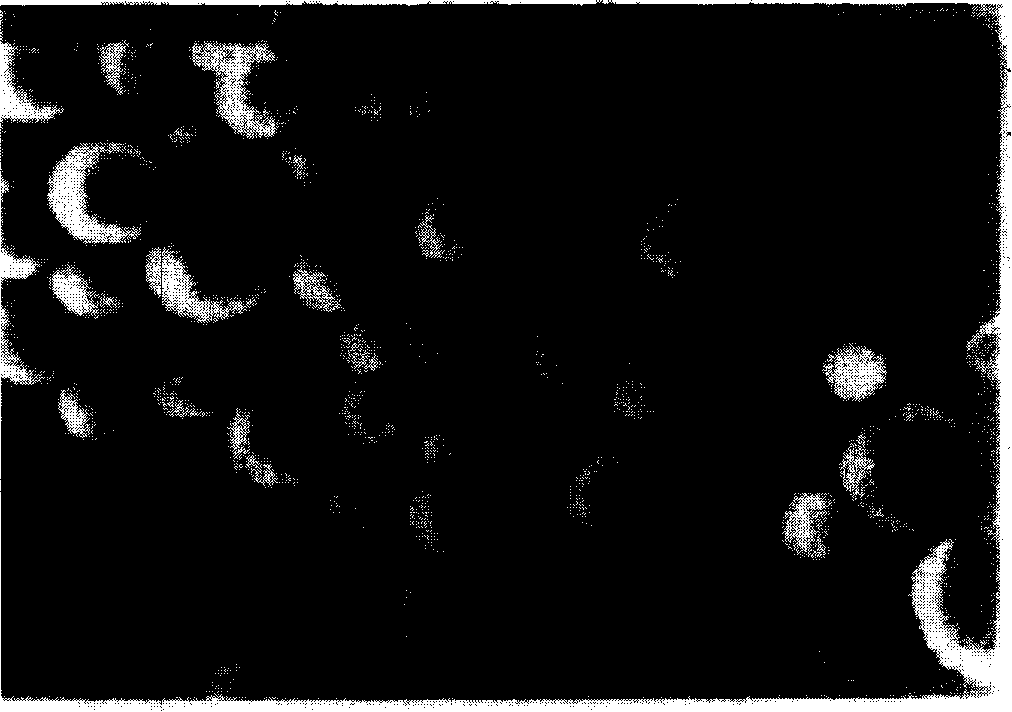
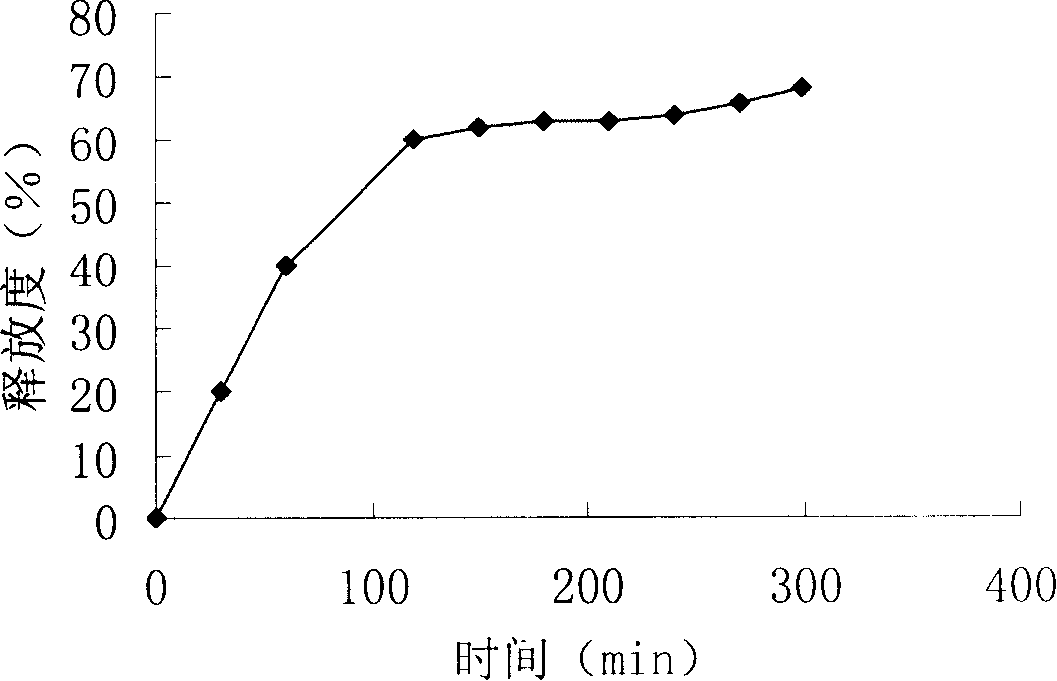
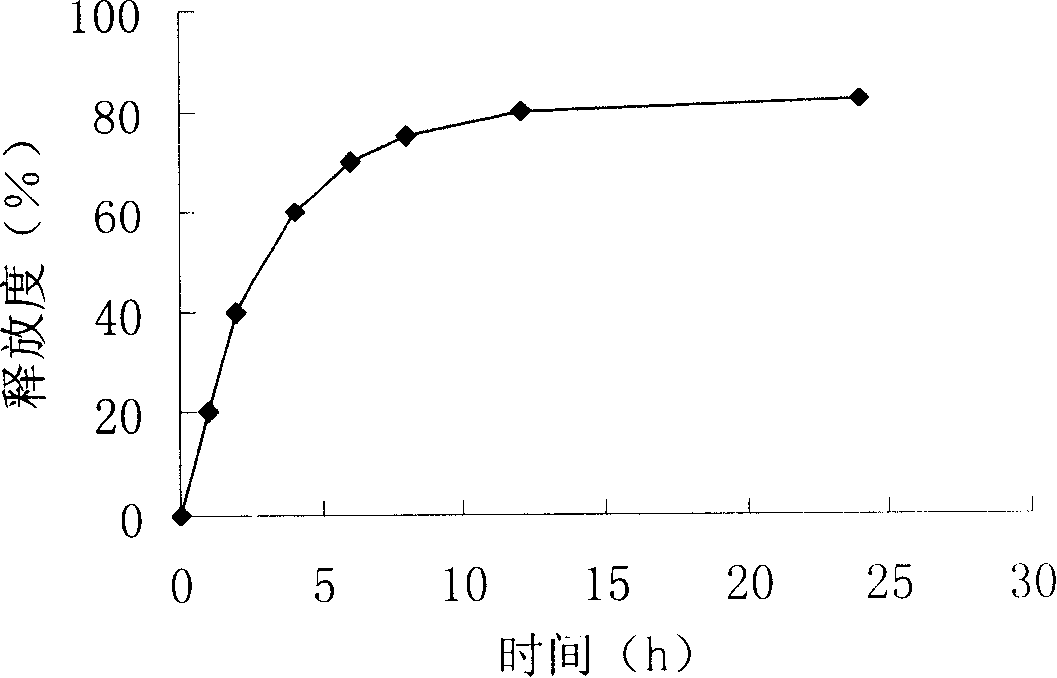
[0045] Suspend 20mg of donepezil in 10ml of 3% chitosan solution, shake and mix evenly, drop into 30ml of corn oil containing 5% span85 and 0.5% tween 80, emulsify at 200rpm for 15min, add dropwise 2ml of 25% glutaraldehyde to solidify . Then add 40 ml of petroleum ether, stir for 5 minutes and then stand still, discard the upper layer, wash the donepezil long-acting sustained-controlled release composition with isopropanol and petroleum ether successively for 3-5 times, and dry in vacuum to obtain a loose powdery solid long-acting sustained-controlled release composition. release composition. figure 1 The appearance of the long-acting sustained and controlled release composition is round and round, and the average particle size is 10.06 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com