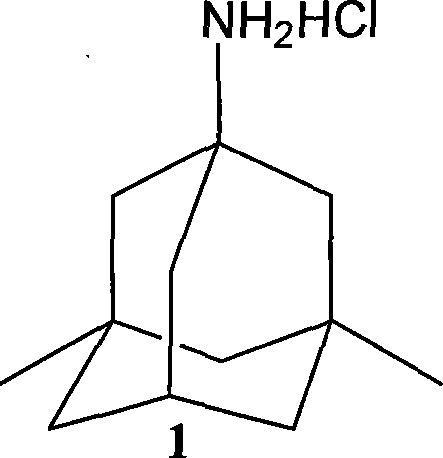
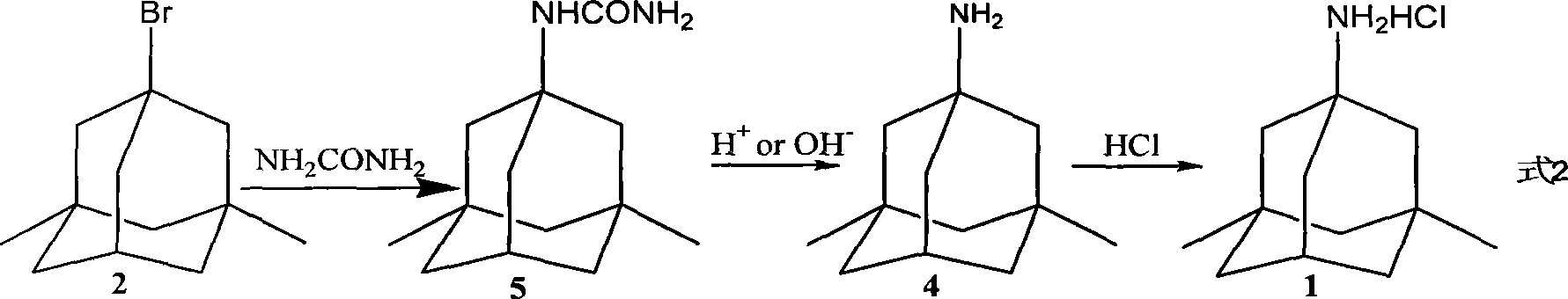Preparation method of memantine salt
A technology for amantadine salt and memantine, which is applied in the field of preparing memantine salt, can solve the problems of difficult control of process operation, product quality, difficulty, many side reactions, etc., achieves cheap raw materials, avoids danger, and simplifies reaction effect of steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
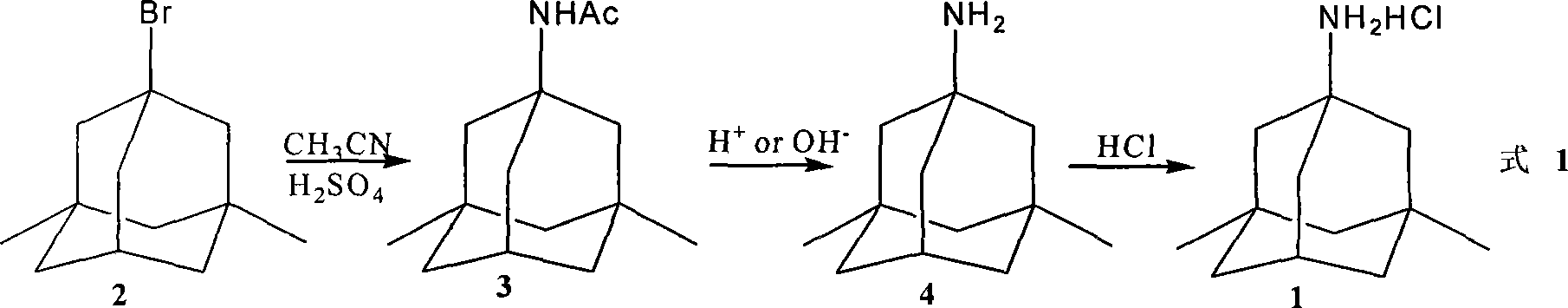
[0032] Preparation of 1-acetamido-3,5-dimethyladamantane:
[0033] 1,3-Dimethyladamantane (82.0g, 0.5mol), acetamide (236.0g, 4mol), Br 2 (640.0g, 4mol) after mixing evenly, stir and react at reflux temperature for 5h. After the reaction is completed, add 200ml of acidified ice water (pH=1), add sodium thiosulfate to remove bromine, and adjust the pH value with 5% NaOH aqueous solution 8 to 9, then extracted twice with ethyl acetate (200mL), combined the ethyl acetate extracts, washed with saturated brine, dried over anhydrous magnesium sulfate, concentrated under reduced pressure to remove the solvent, and the obtained solid was decomposed with ethanol solution Recrystallized and dried to obtain 94.1g white 1-acetamido-3,5-dimethyladamantane, yield: 85.2%, mp: 111-112°C, purity (GC) 99.5%, 1 HNMR (CDCl3, 400Hz) δ: 0.82 (6H, s), 1.13 (2H, m), 1.25 (4H, q, J = 12.5Hz), 1.58 (4H, m, J = 11.4Hz), 1.77 (2H, s), 1.84 (3H, s), 2.11 (1H, t), 5.19 (1H, s), MS (m / z): 221.
[0034] T...
Embodiment 2
[0037] Preparation of 1-carboxamido-3,5-dimethyladamantane:
[0038] 1,3-Dimethyladamantane (16.4g, 0.1mol), formamide (36g, 0.8mol), Br 2 (128g, 0.8mol) after mixing evenly, stir and react at reflux temperature for 12h. After the reaction is completed, add 50ml of acidified ice water (pH=1), add sodium thiosulfate to remove bromine, and adjust the pH value with 5% NaOH aqueous solution 8 to 9, then extracted with ethyl acetate 50mL×2, combined the ethyl acetate extracts, washed with saturated brine, dried over anhydrous magnesium sulfate, concentrated under reduced pressure to remove the solvent, and obtained a yellow viscous liquid without purification , used directly in the next reaction.
[0039] The preparation of memantine hydrochloride:
[0040] Take the reaction solution obtained above, add 50mL of ethanol and 20mL of 36.5% hydrochloric acid, stir and reflux for 6h, the reaction process is monitored by gas chromatography, after the reaction, adjust the pH value to 8-...
Embodiment 3
[0042] Preparation of 1-aminoamido-3,5-dimethyladamantane:
[0043] 1,3-Dimethyladamantane (16.4g, 0.1mol), urea (36g, 0.8mol), Br 2 (128g, 0.8mol) after mixing evenly, stir and react at reflux temperature for 12h. After the reaction is completed, add 50ml of acidified ice water (pH=1), add sodium thiosulfate to remove bromine, and adjust the pH value with 5% NaOH aqueous solution 8 to 9, then extracted with ethyl acetate 50mL×2, combined the ethyl acetate extracts, washed with saturated brine, dried over anhydrous magnesium sulfate, concentrated under reduced pressure to remove the solvent, and obtained a yellow viscous liquid without purification , used directly in the next reaction.
[0044] The preparation of memantine hydrochloride:
[0045] Take the reaction solution obtained above, add 50mL of ethanol and 20mL of 36.5% HCl, stir and reflux for 6h, and monitor the reaction process by gas chromatography. After the reaction, concentrate the solvent to dryness, add 50mL o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com