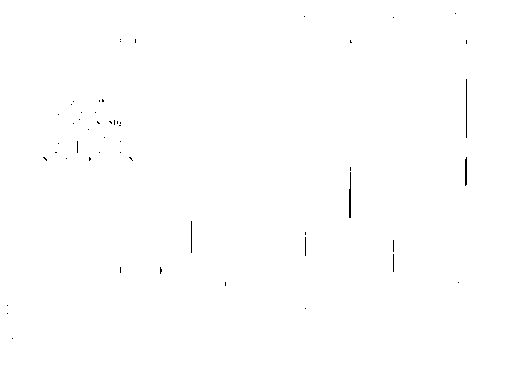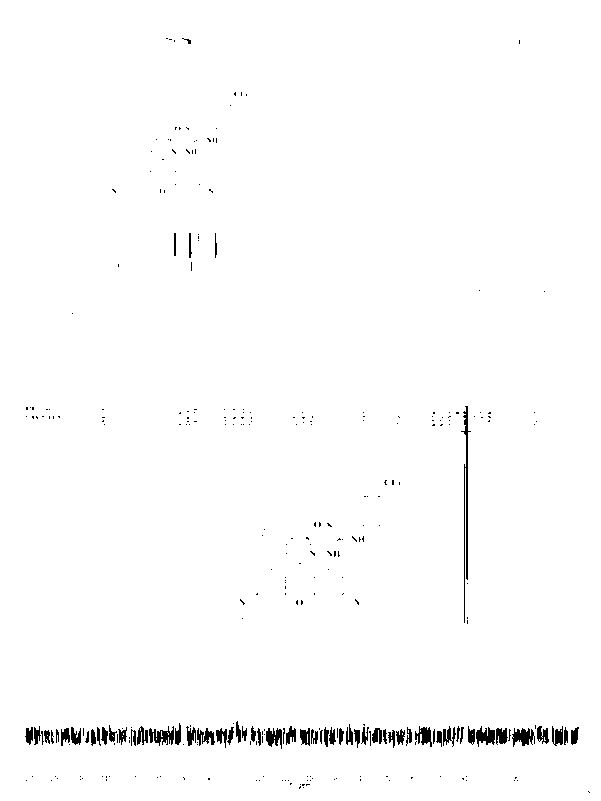Mercury ion fluorescence sensor as well as synthetic method and application thereof
A technology of fluorescence sensor and synthesis method, which is applied in the direction of chemical instruments and methods, fluorescence/phosphorescence, luminescent materials, etc. It can solve the problems of application limitation, interference, long synthesis route of fluorescence sensor, etc., and achieve simple synthesis steps and large-scale production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The synthetic method of mercury ion fluorescent sensor of the present invention, concrete steps comprise as follows:
[0031] The preparation of step (1) intermediate:
[0032] rhodamine B and excess hydrazine hydrate in an organic solvent, control the reaction temperature at 50-120°C, and the reaction time is 24-40h to obtain rhodamine B after hydrazinolysis;
[0033] Wherein, the structural formula of rhodamine B is:
[0034]
[0035] Wherein, the intermediate that step (1) obtains is the structural formula of Rhodamine B after hydrazinolysis is:
[0036]
[0037] Wherein, the organic solvent is ethanol, methanol, dichloromethane, or N,N-dimethylformamide.
[0038] Step (2) preparation of mercury ion fluorescent sensor:
[0039] Dissolve the obtained hydrazinolysis intermediate of rhodamine B in an organic solvent, add an equivalent amount of isocyanate under N2 protection, control the reaction temperature at room temperature, and the reaction time is 24-40h....
Embodiment 1
[0043] The preparation of the hydrazinolysis product (intermediate) of embodiment 1 Rhodamine B:
[0044] In a 250mL glass flask, completely dissolve 20g of rhodamine B in 100mL of ethanol, control the temperature at 100°C, and reflux for 24h. The solvent was removed under reduced pressure, then poured into ice water, filtered and dried. 18.7 g of a white solid was obtained with a yield of 98%. NMR characterization diagram see figure 1 .
[0045] NMR characterization 1H NMR (500MHz, DMSO) δ1.09(t, J=6.7Hz, 12H), 3.31(q, J=19.7Hz, 8H), 4.19(br, 2H), 6.34(br, 4H), 6.38 (br, 2H), 6.98 (d, J=6.5Hz, 1H), 7.46 (t, J=3.6Hz, 2H), 7.75 (d, J=6.7Hz, 1H).
Embodiment 2
[0046] Embodiment 2 Preparation of Mercury Ion Fluorescence Sensor 1 (F1):
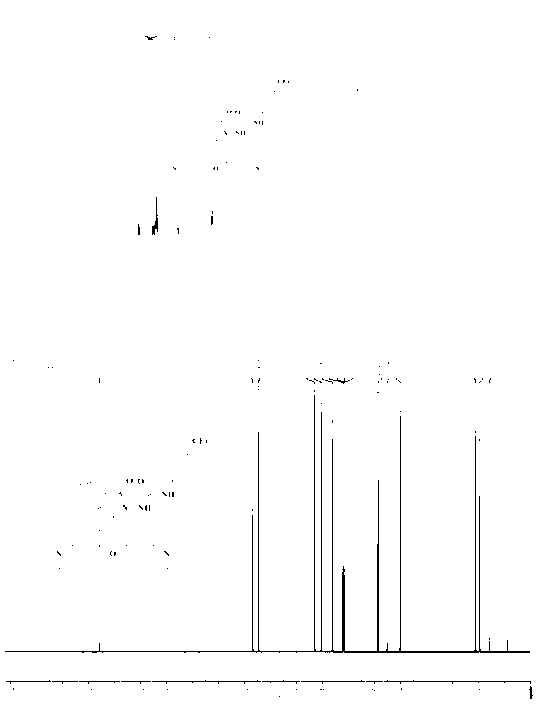
[0047] In a 250mL glass flask, completely dissolve 5g of Rhodamine B in 50mL of N,N-dimethylformamide solution, add an equivalent amount of p-trifluoromethylbenzene isocyanate under N2 protection, and control the temperature at room temperature Stir for 2h. Then the reaction solution was poured into a large amount of ice water, filtered and dried. Obtained F16.8g, yield 97%. F1 NMR characterization diagram and carbon spectrum characterization diagram are shown in figure 2 .
[0048] NMR characterization 1H NMR (500MHz, DMSO) δ0.85(br, 12H), 3.28(q, J=3.4Hz, 8H), 6.32(d, J=5.25Hz, 4H), 6.33(s, 2H), 7.04(d , J=7.25Hz, 1H), 7.46-7.60(m, 6H), 7.85(d, J=7.15Hz, 1H), 8.21(s, 1H).
[0049] Carbon spectrum characterization 13 C NMR (500MHz, DMSO) δ12.9, 44.6, 68.2, 97.7, 106.5, 114.3, 121.9, 124.1, 125.3, 128, 128.3, 128.8, 129.2, 131.3, 139.5, 132.1, 132.6, 142.7, 149.4, 1531.8, , 160.8.
[0050] H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com