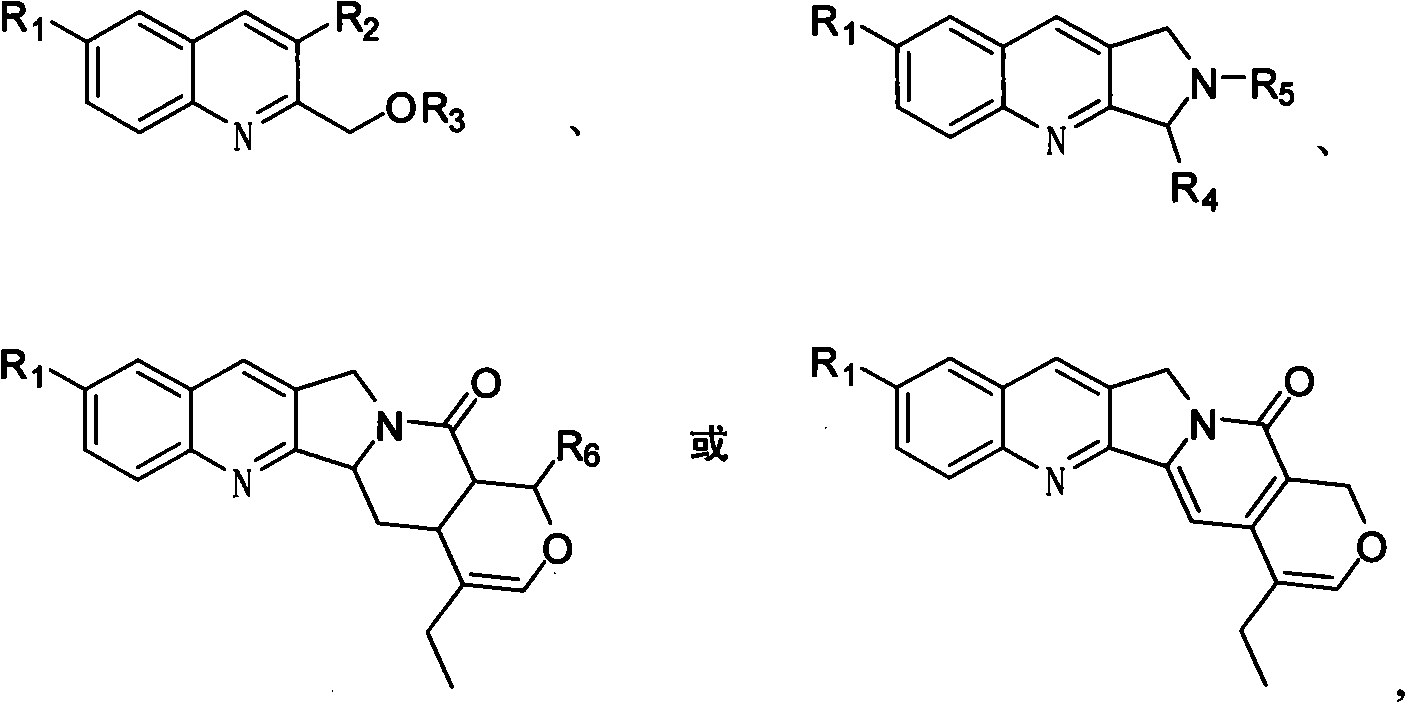
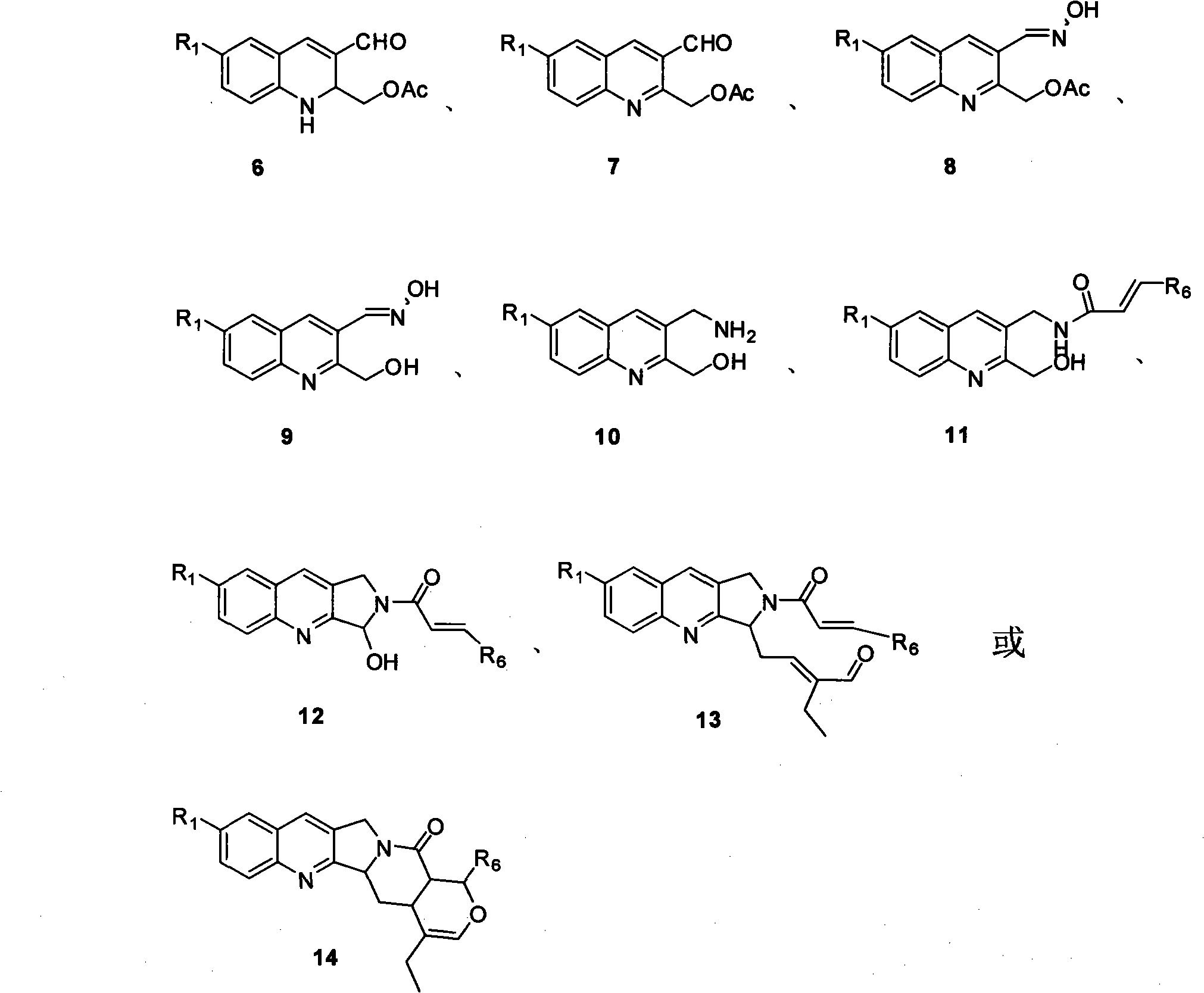
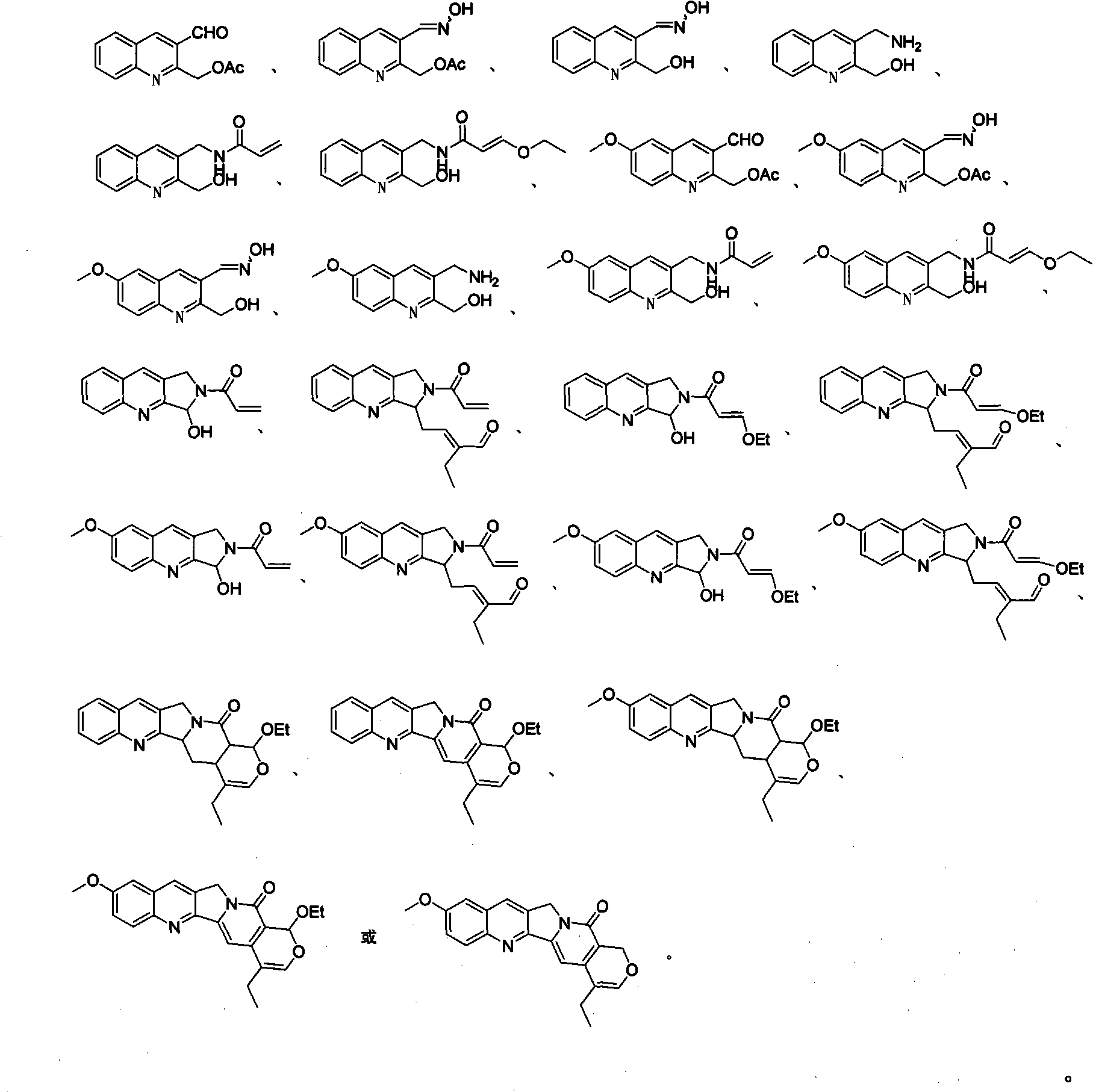Quinoline compounds, synthesizing method, applications in synthesis of alkaloid of camptothecins
A technology of compounds and quinolines, which is applied in the field of organic synthesis, can solve problems such as not being practical and in great demand
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050]
[0051] In compound 5 (5g, 39mmol) dichloromethane solution, add molecular sieves, catalytic amount of pyrrolidine (0.32mL, 3.9mmol) and benzoic acid (0.32mL, 3.9mmol), after stirring for five minutes, add compound 4 (7.09g , 58.57mmol), stirred at room temperature for about half an hour. After the reaction was completed, the molecular sieves were filtered off, silica gel was added to the filtrate, and the mixture was stirred overnight at room temperature. Silica gel was filtered off, concentrated, and column chromatography gave product 6a (R 1 =H, 8.44 g, 82%).
[0052] 1 H NMR (CDCl3, 300MHz): δ9.52(s, 1H), 7.28(d, 1H, J=4.8Hz), 7.15(t, 1H, J=7.9Hz), 7.10(d, 1H, J=7.5 Hz), 6.67(t, 1H, J=7.4Hz), 6.52(d, 1H, J=8.1Hz), 4, 82(m, 1H), 4.67(s, 1H), 4.04(m, 2H), 1.94(s, 3H),
[0053] According to the same operation, compound 6b (R 1 =OMe, 84%).
[0054] 1 H NMR (CDCl3, 300MHz): δ9.53(s, 1H), 7.25(s, 1H), 6.81(dd, 1H, J=8.7, 3.0Hz), 6.66(d, 1H, J=3.3Hz), 6.50(d, ...
Embodiment 2
[0056]
[0057] At room temperature, the activated manganese dioxide (13.2g, 151.5mmol) was added to the dichloromethane solution of compound 6 (7.0g, 30.3mmol) at one time, and stirred at room temperature until the raw material disappeared. Filtration, concentration, column chromatography, product 7a (R 1 =H, 6.2 g, 89%).
[0058] 1 H NMR (300MHz, CDCl 3 ): δ10.31(s, 1H), 8.69(s, 1H), 8.16(d, 1H, J=8.7Hz), 8.00(d, 1H, J=7.8Hz), 7.90(m, 1H), 7.67 (m, 1H), 5.75(s, 2H), 4, 82(m, 1H), 2.24(s, 3H).
[0059] According to the same operation, compound 7b (R 1 =OMe, 87%).
[0060] 1 H NMR (300MHz, CDCl 3 ): δ10.31(s, 1H), 8.69(s, 1H), 8.45(d, 1H, J=9.3Hz), 7.52(dd, 1H, J=7.8, 2.7Hz), 7.22(d, 1H, J=2.1Hz), 5.70(s, 2H), 3.97(s, 3H), 2.20(s, 3H).
Embodiment 3
[0062]
[0063] Add hydroxylamine hydrochloride (2.54g, 36.5mmol) and sodium acetate (2.99g, 36.5mmol) successively to the ethanol solution of compound 7 (5.57g, 24.3mmol), and stir at room temperature for about ten minutes. After spinning off most of the ethanol, water was added, extracted with dichloromethane, dried over anhydrous sodium sulfate, concentrated, and directly put into the next step.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com