Preparation method of 2,5-dichlorophenol
A technology of dichlorophenol and p-dichlorobenzene, which is applied in the field of preparation of 2,5-dichlorophenol, can solve the problems of not being suitable for large-scale industrial production, difficult separation of mixed phenols, and low conversion rate of raw materials, etc., to achieve suitable Large-scale industrial production, less waste production and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
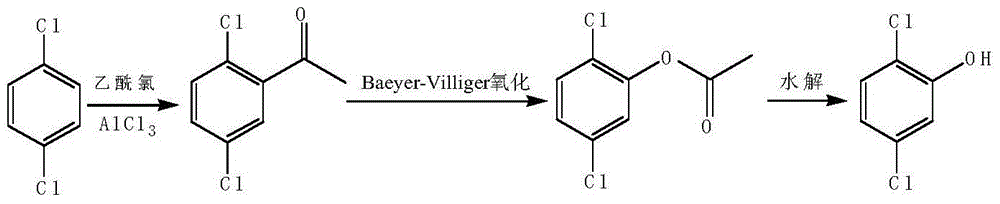
[0021] (1) Add 14.7g of p-dichlorobenzene and 26.6g of anhydrous aluminum trichloride to a 250ml three-neck flask, heat up to 65°C while stirring, add 8.6g of acetyl chloride dropwise, heat up to 115°C after 2 hours, and continue the reaction 6h. Slowly add 50ml of ice water, then add 100ml of dichloromethane for extraction, separate the dichloromethane phase and distill out the dichloromethane, carry out vacuum distillation with an oil pump, collect the fractions at about 70°C, and obtain 2,5-dichlorophenethyl The ketone was 14.2g, the yield was 75%, and the content detected by gas chromatography was 99%.
[0022] (2) Add 60 ml of a mixed solvent of dichloromethane and acetone with a volume ratio of 2:1 to a 250 ml three-necked flask at room temperature, then add 2.46 g of scandium trifluoromethanesulfonate as a catalyst, and 50 ml of 30% peracetic acid as an oxidizing agent. After stirring evenly, 18.9 g of 2,5-dichloroacetophenone was added dropwise, and the dropwise addit...
Embodiment 2
[0025] (1) Add 14.7g of p-dichlorobenzene and 33.3g of anhydrous aluminum trichloride to a 250ml three-neck flask, heat up to 65°C while stirring, add 11.8g of acetyl chloride dropwise, and heat up to 100°C after 2 hours of dripping, continue the reaction 5h. Slowly add 50ml of ice water, then add 100ml of dichloromethane for extraction, separate the dichloromethane phase and distill out the dichloromethane, carry out vacuum distillation with an oil pump, collect the fractions at about 70°C, and obtain 2,5-dichlorophenethyl The ketone was 18.0 g, the yield was 95%, and the content detected by gas chromatography was 99%.
[0026] (2) Add 105ml of a mixed solvent of dichloromethane and acetone at a volume ratio of 20:1 to a 250ml three-necked flask at room temperature, add 2.46g of scandium trifluoromethanesulfonate as a catalyst, and 46g of 75% m-chloroperoxybenzoic acid as an oxidizing agent. After stirring evenly, 18.9 g of 2,5-dichloroacetophenone was added dropwise, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com