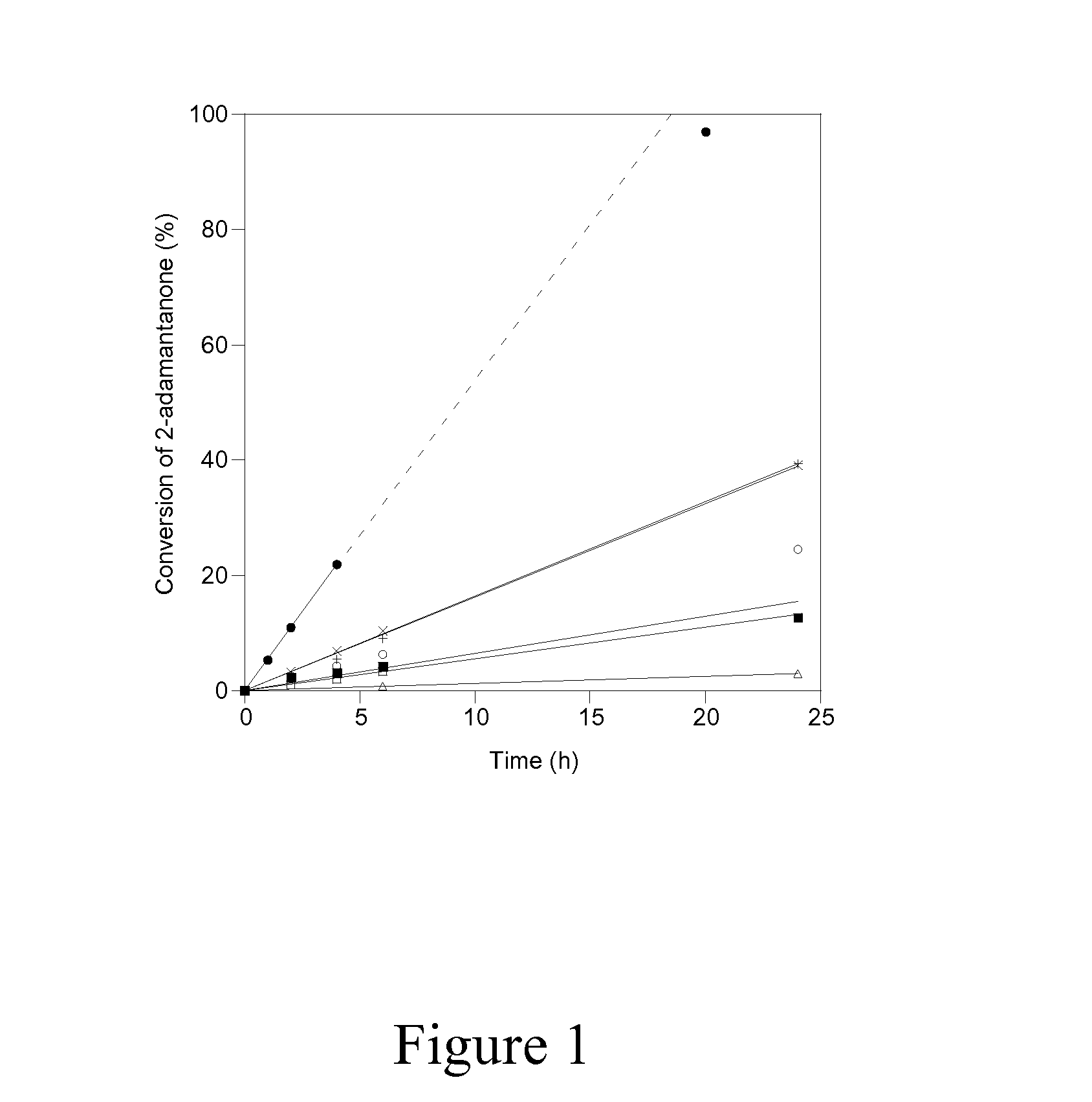
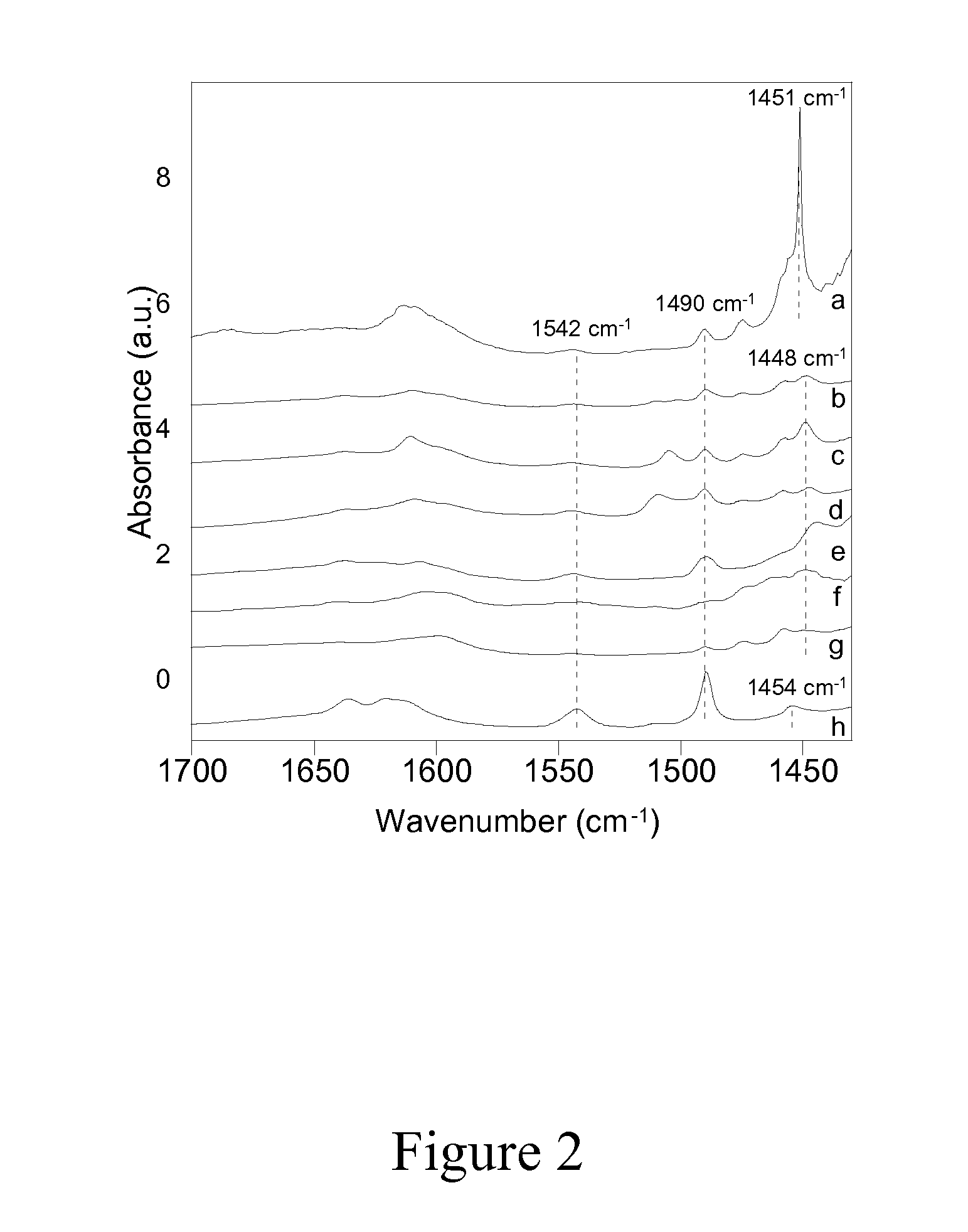
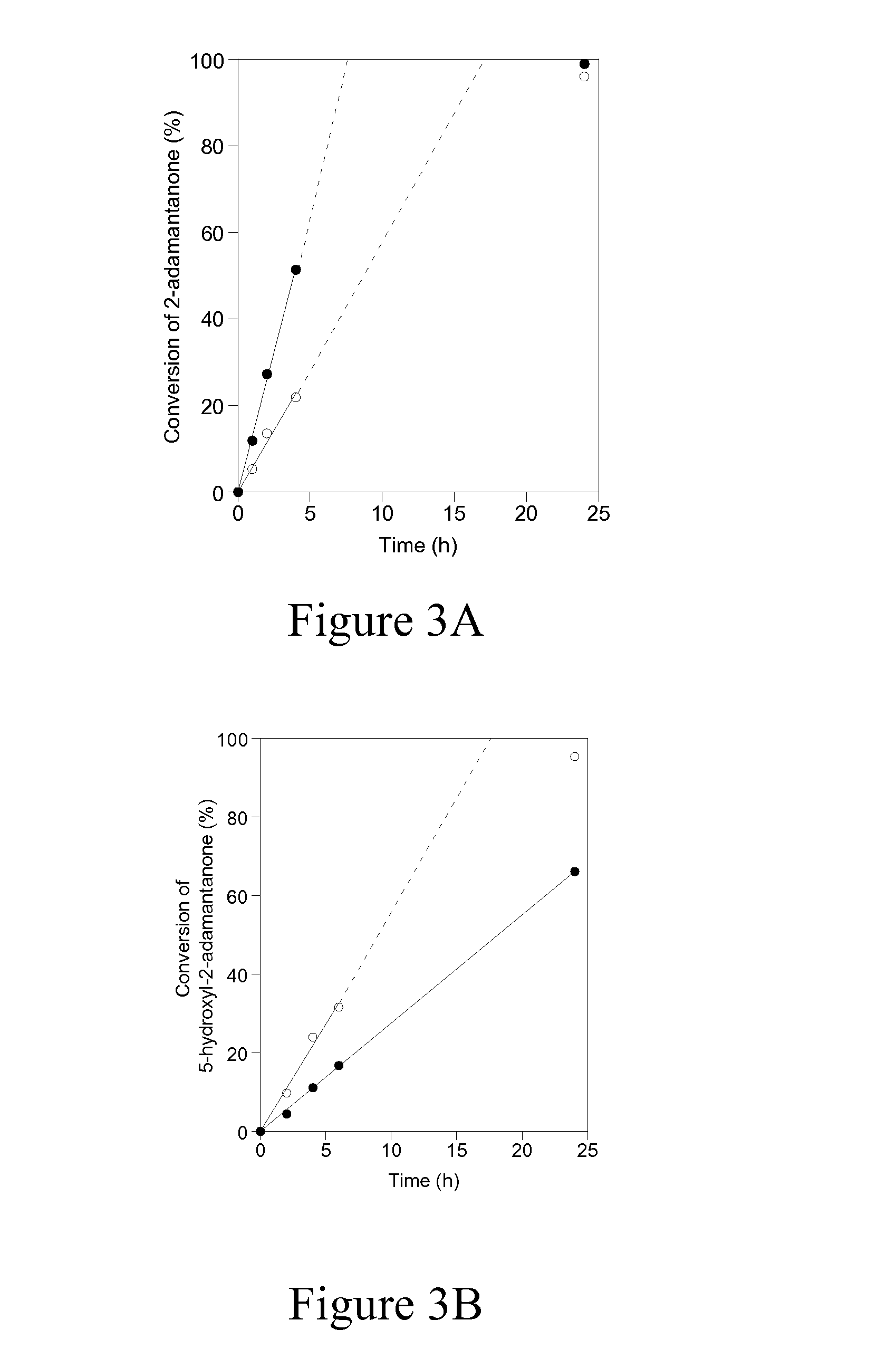
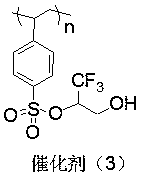
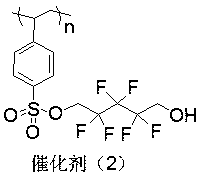
Patents
Literature
38 results about "Baeyer–Villiger oxidation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
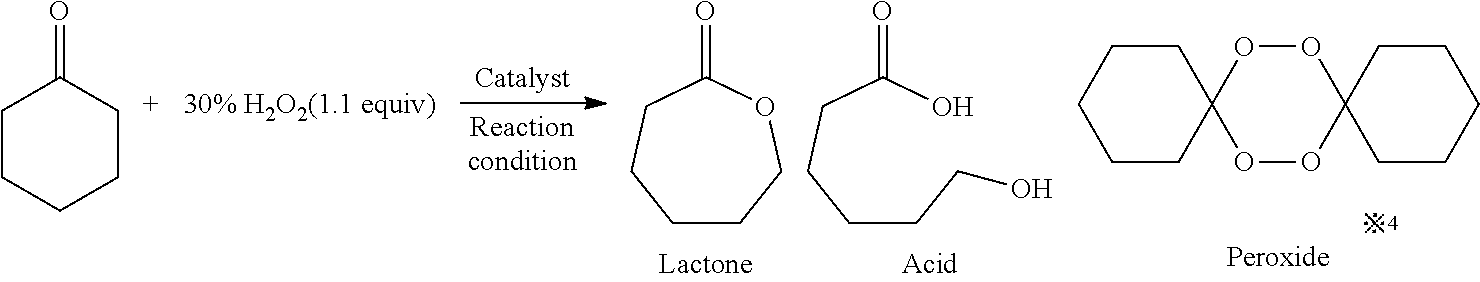
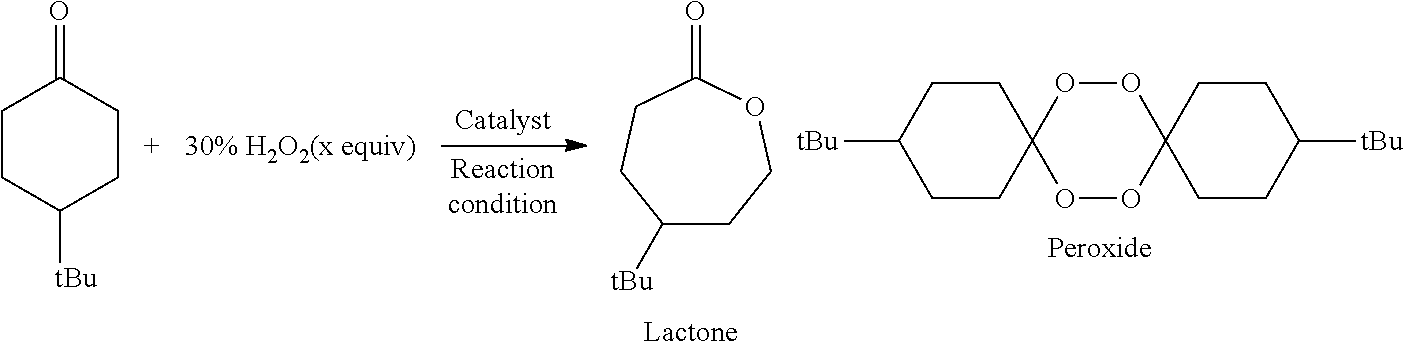
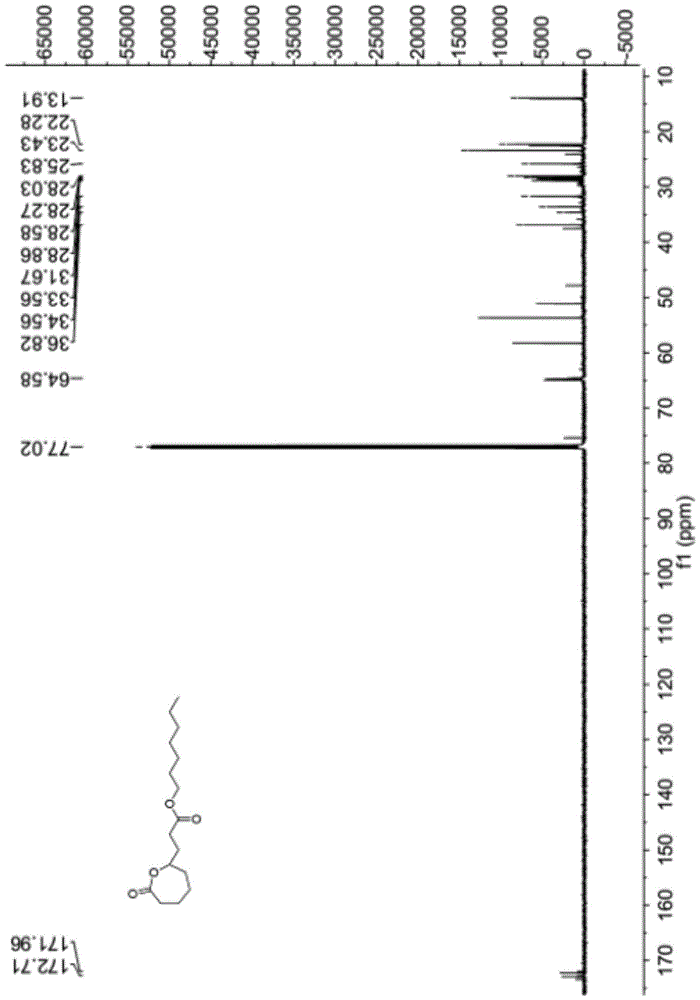
The Baeyer–Villiger oxidation is an organic reaction that forms an ester from a ketone or a lactone from a cyclic ketone, using peroxyacids or peroxides as the oxidant. The reaction is named after Adolf von Baeyer and Victor Villiger who first reported the reaction in 1899.
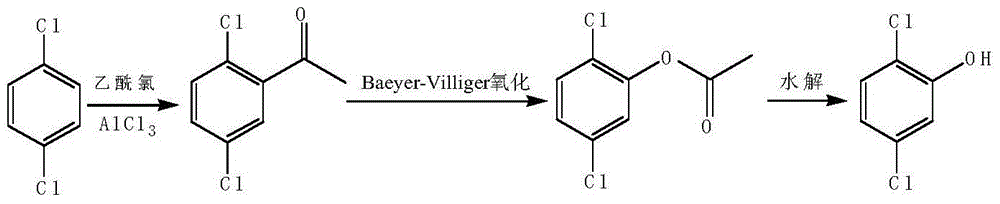
Preparation method of 2,5-dichlorophenol
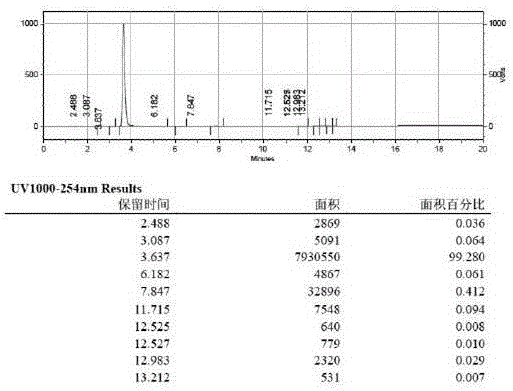
InactiveCN104591973AThe synthesis process is simpleShort routeOrganic compound preparationCarboxylic acid esters preparationHydrolysisPeroxide
The invention discloses a preparation method of 2,5-dichlorophenol and relates to the technical field of pesticide intermediate synthesis. The preparation method comprises the following steps: with p-dichlorobenzene as a start raw material, performing a Friedel-Crafts acylation reaction between the p-dichlorobenzene and acetyl chloride in the presence of aluminum trichloride to obtain 2,5-dichloroacetophenone; performing a Baeyer-Villiger oxidation reaction between the 2,5-dichloroacetophenone and a peroxide in the presence of a catalyst at room temperature to obtain 2,5-dichlorobenzene acetate; and performing a hydrolysis reaction between the 2,5-dichlorobenzene acetate and inorganic aqueous alkali in a reflux condition to obtain 2,5-dichlorophenol. The preparation method disclosed by the invention has the characteristics of simple synthesis process, short line, low production cost and high yield; and moreover, with less quantity of generated three wastes and high environmental protection property, the preparation method is more suitable for large-scale industrial production.
Owner:ANHUI XUELANG BIOTECHNOLOGY CO LTD
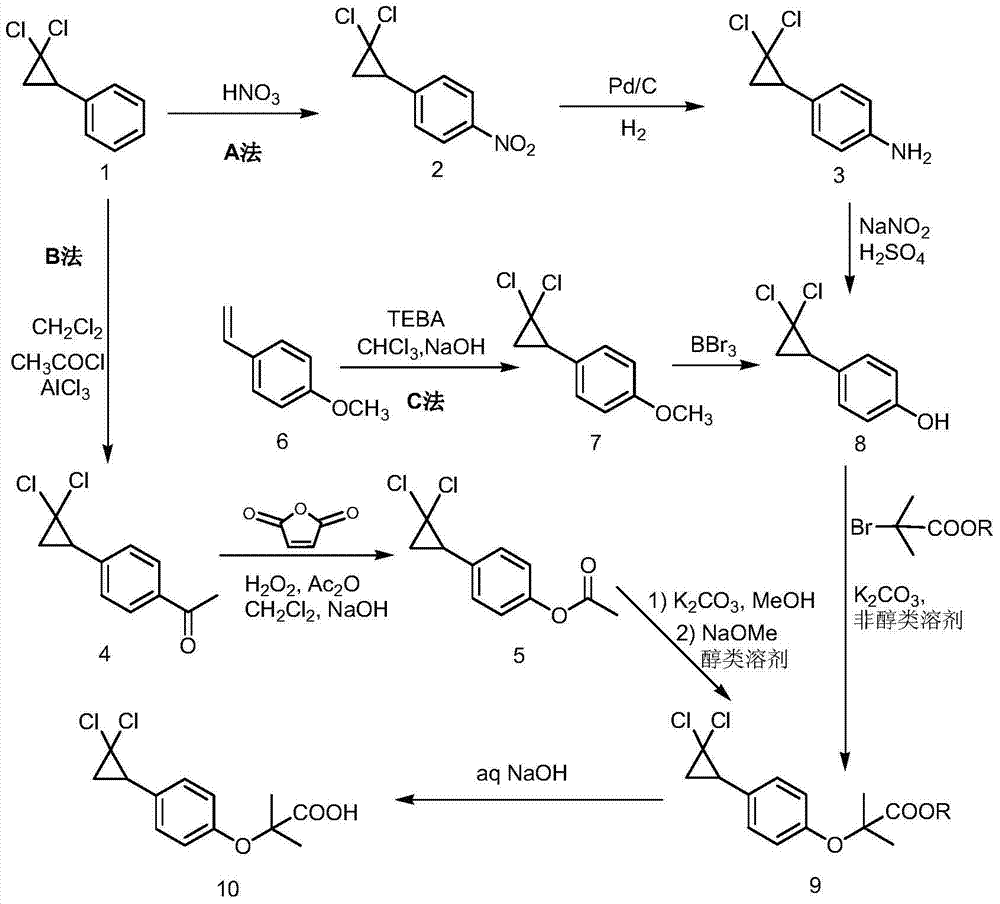
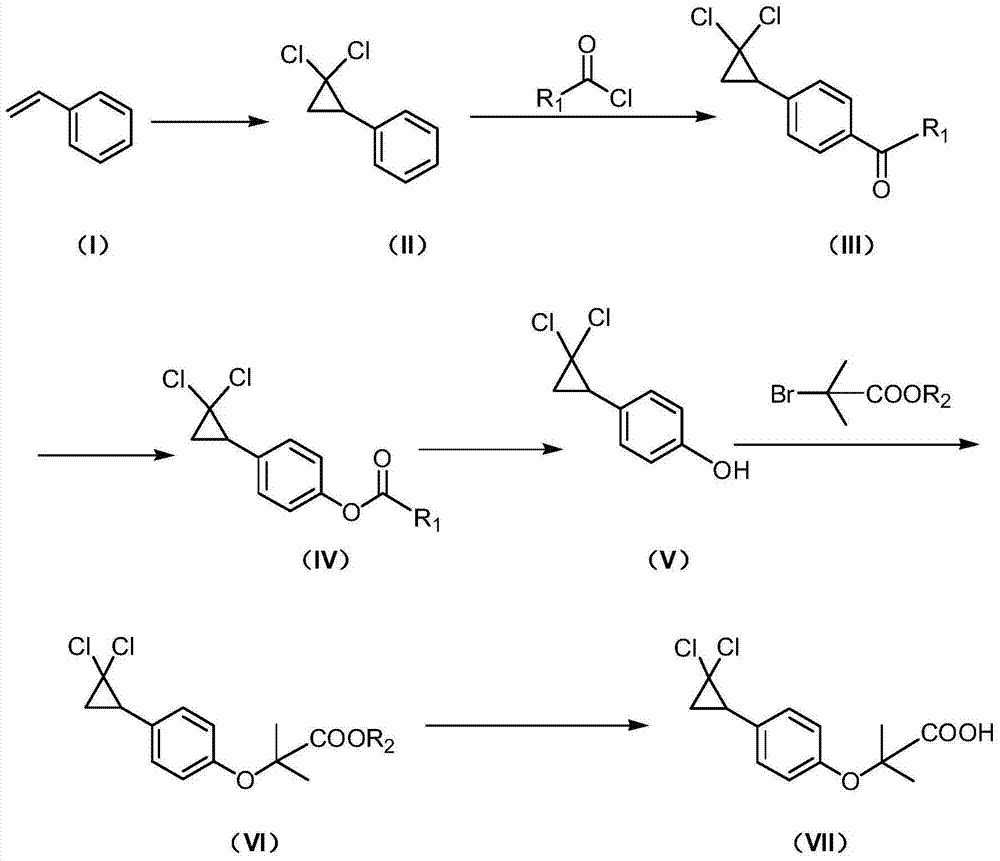
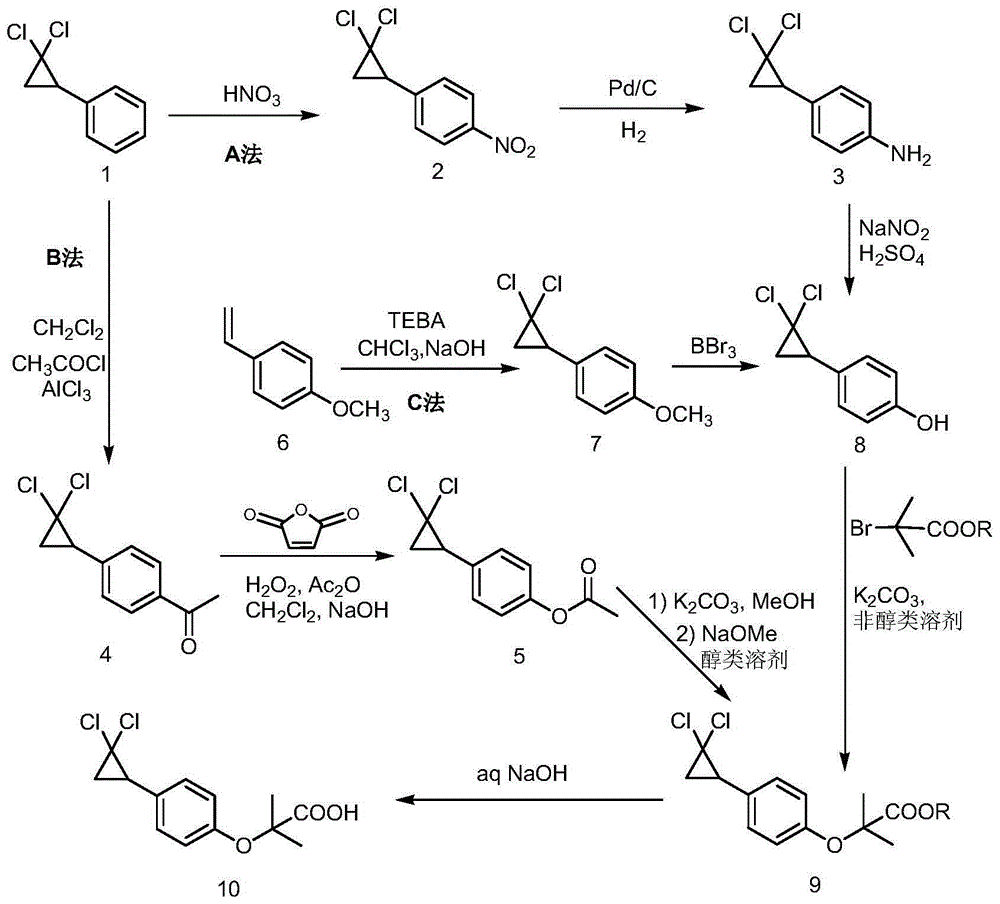
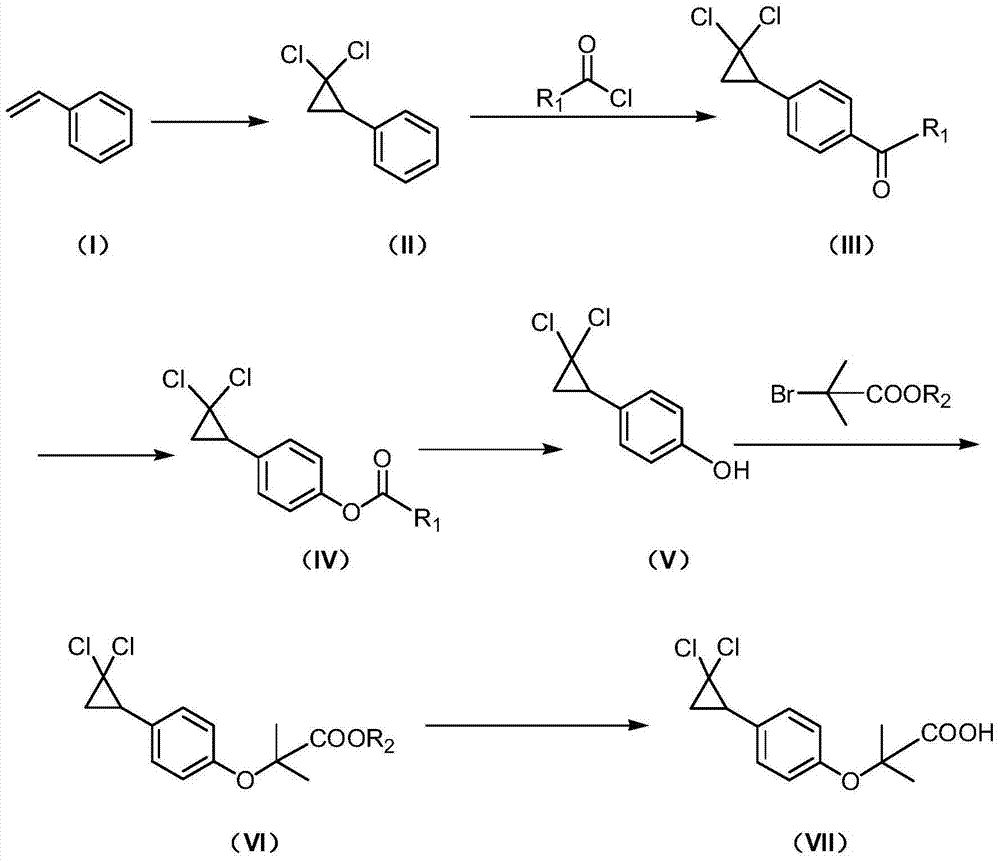
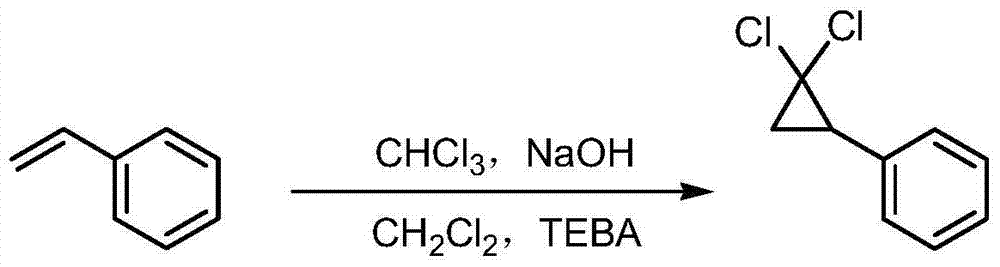
Synthetic method of ciprofibrate
ActiveCN103613498AAvoid generatingHigh yieldOxygen-containing compound preparationOrganic compound preparationAcetic anhydrideOrtho position
The invention discloses a synthetic method of ciprofibrate. The method comprises the following steps: taking styrene as a starting material; obtaining the ciprofibrate by the processes of cyclization, acylation, Baeyer-Villiger oxidation, alcoholysis, alkylation and hydrolysis, wherein an acylation reagent adopted in the acylation process is R1COCl; the R1 is C4-C12 alkyl. Fatty acyl chloride of which a hydrocarbyl structure is a long chain is adopted in acylation reaction, so that the steric hindrance is increased, the reaction activity of acyl chloride is reduced, the content of the produced ortho-position isomer is smaller than 0.2% and superior to that of acetyl chloride reaction (the content of the ortho-position isomer is 0.5-1%), the yield and the purity of the product are improved, and meanwhile, a room-temperature reaction condition is adopted, so that the energy consumption of production is reduced. Urea peroxide instead of hydrogen peroxide is used as an oxidant in the Baeyer-Villiger oxidation, and acetic acid is used instead of acetic anhydride, so that the reaction condition is milder, and the operation is more controllable.
Owner:ZHEJIANG SANMEN HYGECON PHARMA CO LTD
Highly active, selective, accessible, and robust zeolitic sn-baeyer-villiger oxidation catalyst
Provided is a process of conducting a Baeyer-Villiger oxidation which comprises contacting a ketone and an oxidant in the presence of an Sn-DZ-1 catalyst to thereby oxidize the ketone to an ester. The Sn-DZ-1 catalyst comprises Sn heteroatoms on the external surface of the zeolitic material lattice framework, and B heteroatoms, or silanols created from boron hydrolysis, throughout the remainder of the lattice framework.
Owner:RGT UNIV OF CALIFORNIA +1
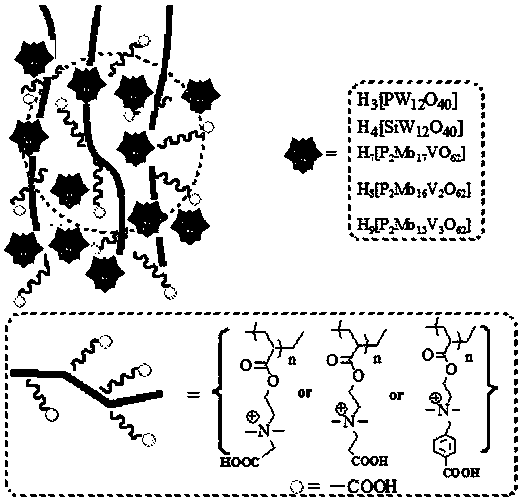
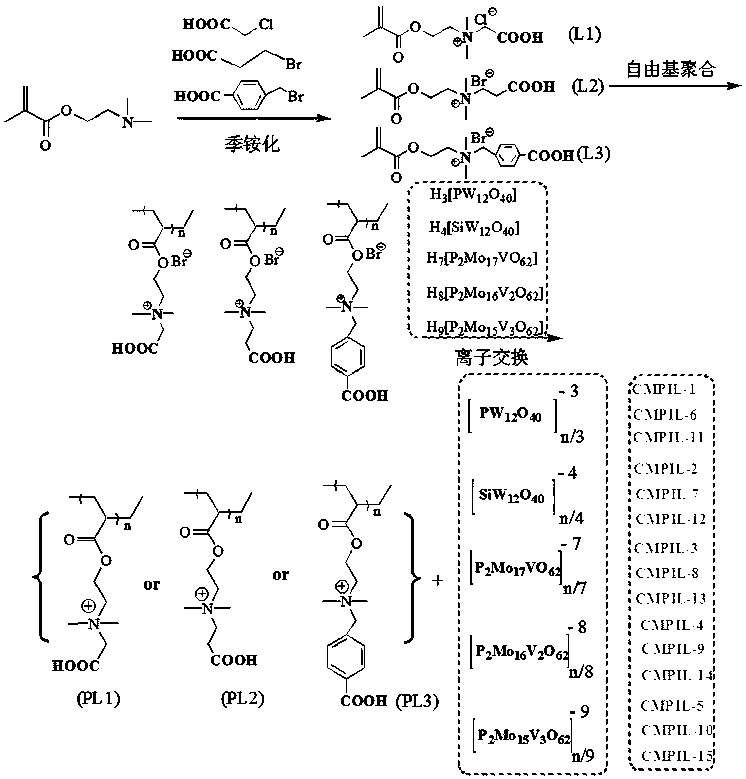
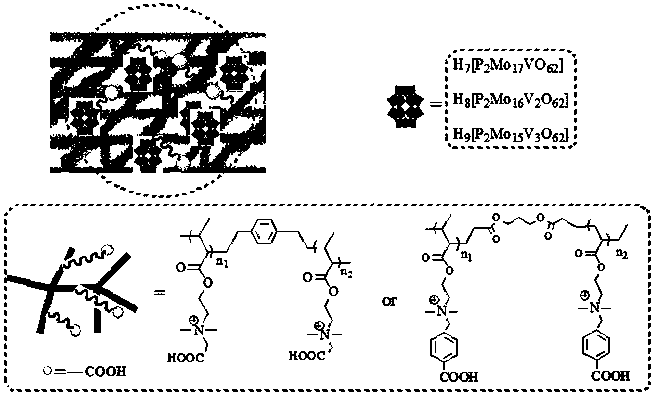
Carboxyl functionalized porous heteropoly acid polyionic liquid and use thereof
ActiveCN109289919AFacilitate the reaction between two phasesSeparation in timeOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsCross-linkVanadium doping
The invention discloses a carboxyl functionalized porous heteropoly acid polyionic liquid and a use thereof. A carboxyl functionalized polyquaternary ammonium salt with a long chain linear structure and a heteropoly acid as building units are self-assembled into a three-dimensional network porous structure under electrostatic interaction so that the polyionic liquid is obtained, or a carboxyl functionalized polyquaternary ammonium with a long chain cross-linked structure and a Dawson-type vanadium-doped heteropoly acid as building units are self-assembled into a three-dimensional network porous structure under electrostatic interaction. The carboxyl functionalized porous heteropoly acid polyionic liquid can be used as a heterogeneous catalyst. Industrial grade hydrogen peroxide is used asan oxidant. A cyclic ketone compound undergoes Baeyer-Villiger oxidation to produce a corresponding cyclic lactone under the solventless condition. The catalytic system has a novel structure, is usedfor a Baeyer-Villiger oxidation reaction, and has the characteristics of good selectivity, mild reaction condition, large operation flexibility and convenient recycling of the catalytic system.
Owner:MINJIANG UNIV
Synthesis method of 1-pyrenol and intermediates thereof
ActiveCN105732331AHigh reaction yieldReduce pollutionOrganic compound preparationCarboxylic acid esters preparationBaeyer–Villiger oxidationAcetyl chloride
The invention discloses a synthesis method of 1-pyrenol and intermediates thereof.The synthesis method includes firstly, preparing an intermediate, namely acetylpyrene by subjecting pyrene and acetyl chloride to Friedel-Crafts reaction; secondly, preparing an intermediate, namely acetoxypyrene by Baeyer-Villiger oxidation rearrangement; thirdly, preparing the 1-pyrenol by saponification.The synthesis method has the advantages that the 1-pyrenol and the intermediates thereof are prepared from the pyrene and the acetyl chloride, and accordingly the synthesis method is economic, easy to operate, little in environmental pollution, capable of saving production cost for enterprises and high in reaction yield rate of the 1-pyrenol and the intermediates thereof.
Owner:YURUI SHANGHAI CHEM
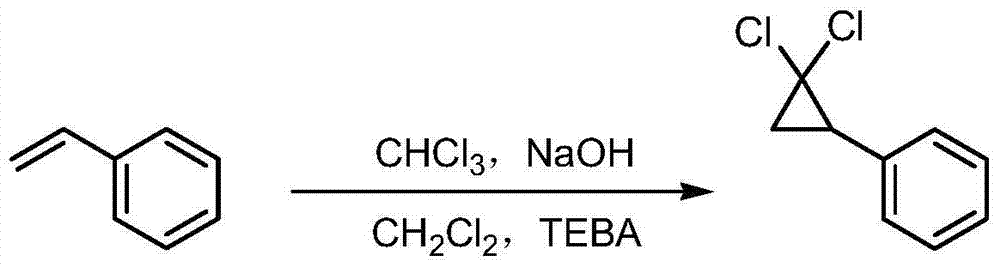
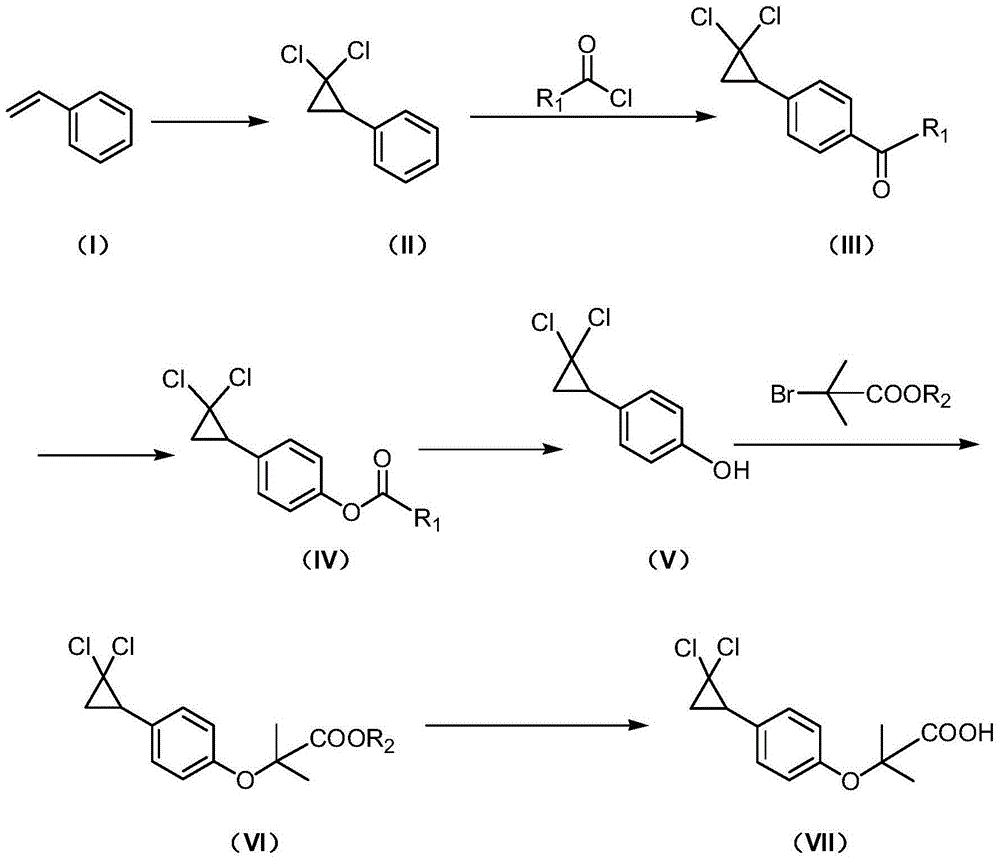
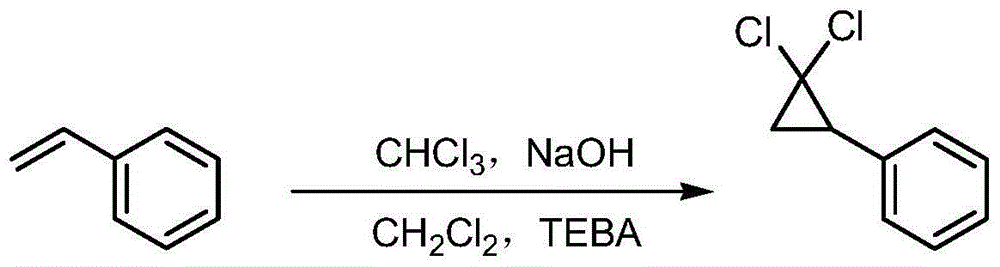
Method for synthesizing ciprofibrate intermediate and the intermediate
InactiveCN104909994AAvoid generatingHigh yieldOrganic compound preparationCarbonyl compound preparation by condensationBaeyer–Villiger oxidationAcetyl chloride
The invention discloses a method for synthesizing ciprofibrate intermediate. The method comprises: conducting cyclization reaction on styrene to obtain 2,2-dichloro-cyclopropylmethyl phenyl; conducting Friedel-Crafts acylation reaction on 2,2-dichloro-cyclopropylbenzene to obtain a compound (III), wherein an acylating agent is R1COCl, and R1 is C4-C12 alkyl group; and conducting Baeyer-Villiger oxidation reaction on the compound (III) to obtain a compound (IV). The invention employs fatty acyl chloride with long chain hydrocarbyl structure in the acylation reaction to increase steric hindrance and reduce the reaction activity of the acyl chloride; the content of the produced ortho isomer is less than 0.2%; and the method improves the yield and purity of the product, is superior to the reaction of acetyl chloride (the content of ortho isomer is 0.5-1%), and the utilization of room temperature conditions reduces energy consumption for production.
Owner:ZHEJIANG SANMEN HYGECON PHARMA CO LTD
Method for manufacturing ester
InactiveUS20130217898A1High yieldHigh catalytic activityOrganic oxidationOrganic compound preparationCyclohexanoneAryl
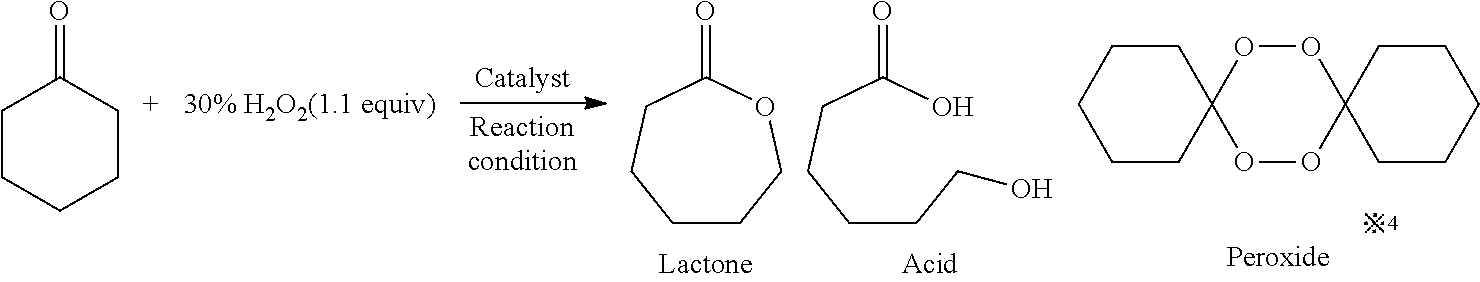
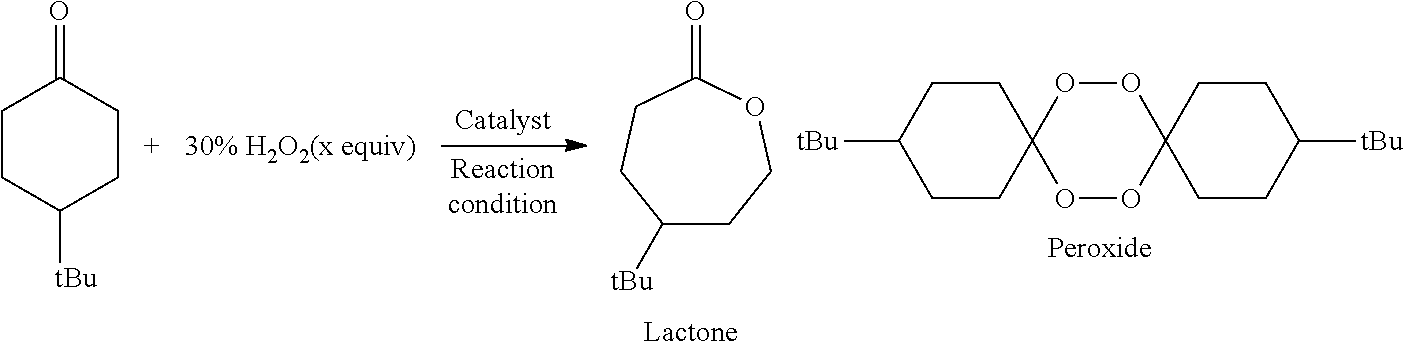
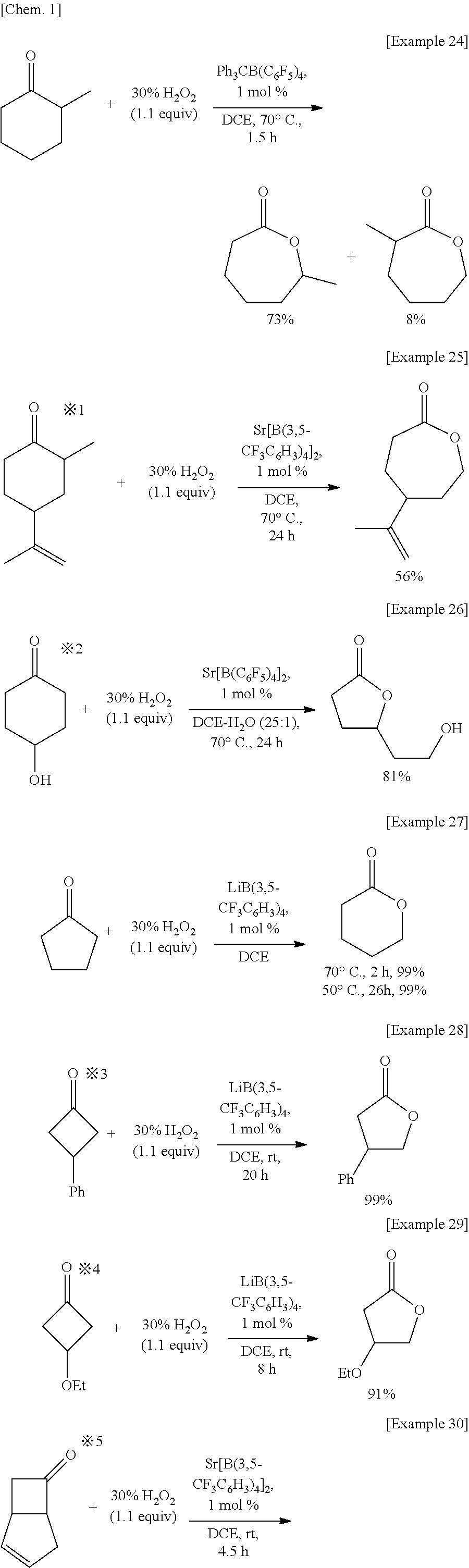
The present invention relates to a method for manufacturing an ester from a ketone or an aldehyde, which is a reactive substrate, by a Baeyer-Villiger oxidation reaction using hydrogen peroxide, and in this method, as a catalyst, M(BAr4)n, which is a metal borate, is used (M represents an alkali metal or an alkaline earth metal; Ar represents an aryl; and n is the same number as the valence of M). For example, when cyclohexanone was used as the reactive substrate, and Sr[B(3,5-CF3C6H3)4]2 was used as the catalyst, ε-caprolactone was obtained at an isolated yield of 82%.
Owner:NAGOYA UNIVERSITY
Preparation method with four-step synthesis of 28-homobrassinolide
ActiveCN108727462ANo pollution in the processRaw materials are easy to getOrganic chemistry methodsSteroidsEnvironmental resistanceBaeyer–Villiger oxidation
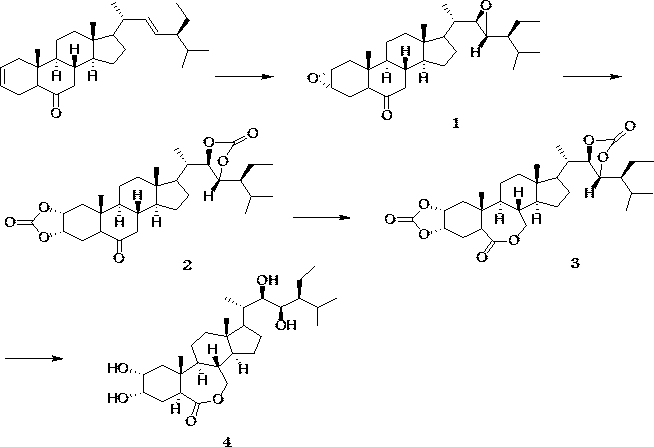
The invention discloses a preparation method with four-step synthesis of 28-homobrassinolide, which takes (2,22)-diene-24S-ethyl-5alpha-cholest-6-ketone as a raw material, and 28-homobrassinolide is obtained from epoxidation, esterification, Baeyer-Villiger oxidation and hydrolysis reaction, the preparation method provided by the invention has the advantages of green environmental protection, no pollution, environment friendliness, easily available raw materials, wide source, low cost, simple method and suitability for industrial production, and the preparation method solves the problems of high preparation cost and difficulty in industrial production in the prior art.
Owner:JIANGXI AGRICULTURAL UNIVERSITY +1
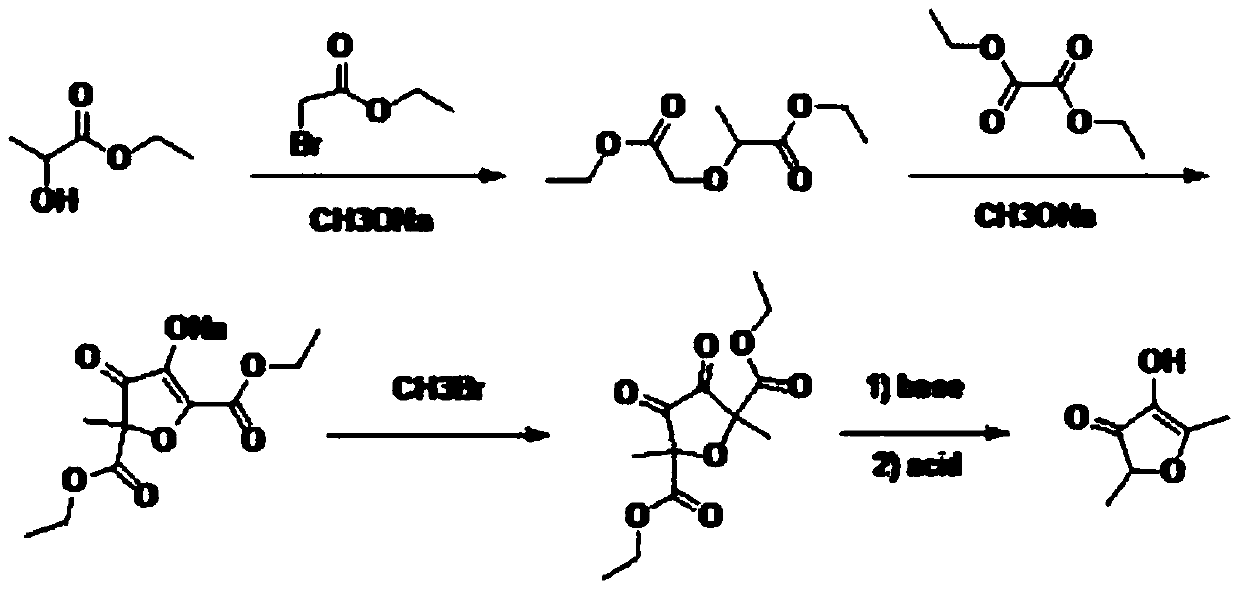
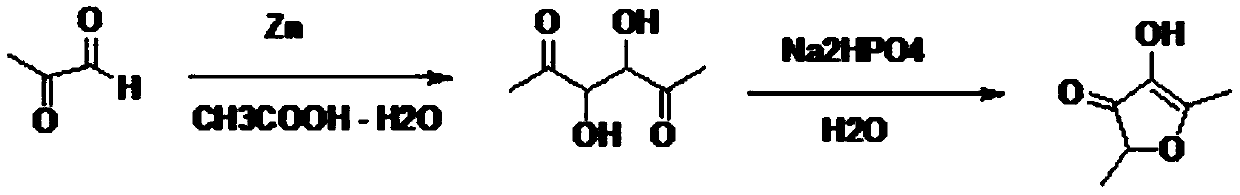
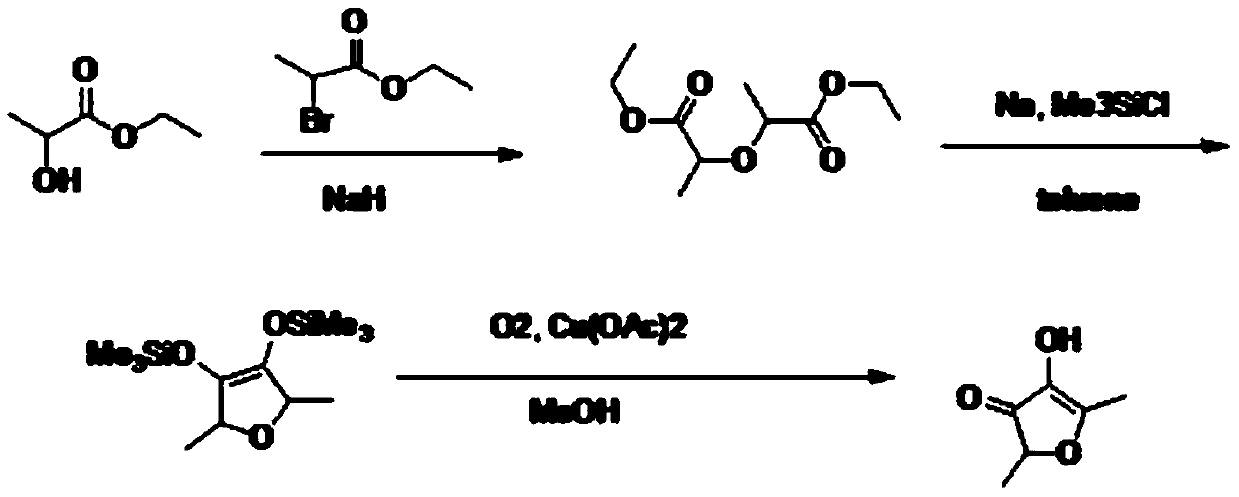
Preparation method of 2,5-dimethyl-4-hydroxyl-3(2H)furanone
PendingCN109824633ARaw materials are easy to getHigh yieldOrganic chemistryFuranBaeyer–Villiger oxidation
The invention discloses a preparation method of 2,5-dimethyl-4-hydroxyl-3(2H)furanone. The preparation method comprises following three steps: (1) taking an easily available 3,4-diacetyl-2,5-hexanedione as the primary raw material, and carrying out intra-molecular dehydration to close the ring to obtain 2,5-dimethyl-3,4-diacetyl furan in the presence of a catalyst; (2) oxidizing 2,5-dimethyl-3,4-diacetyl furan through Baeyer-Villiger oxidation to obtain 2,5-dimethyl-3,4-diacetoxyl furan; and (3) hydrolyzing 2,5-dimethyl-3,4-diacetoxyl furan to obtain 2,5-dimethyl-4-hydroxyl-3(2H)furanone. Theused raw material 3,4-diacetyl-2,5-hexanedione is prepared through coupling or electrochemical coupling of acetyl acetone under the action of NBS and an alkali; the raw material preparation is simple, the raw material is easily available, at the same time, the synthesis route is short, the yield is high, the synthesis conditions are mild, the operation is simple, the purification method is simple, the yield is high, no toxic gas such as hydrogen bromide is generated, and the generated wastewater, waste solids, and waste gas are little.
Owner:方国华
Continuous synthesis method of milk lactone perfume
InactiveCN109678699AHigh yieldIncrease profitOrganic compound preparationPreparation from carboxylic acid esters/lactonesCyclohexanoneDistillation
The invention belongs to the technical field of synthetic perfumes, and specifically relates to a continuous synthesis method of milk lactone perfume. The continuous synthesis method comprises the following steps: carrying out aldol reaction under the action of alkaline water, then carrying out hydrogenation reaction and Baeyer-Villiger oxidation, carrying out acidic continuous hydrolysis, and dehydrating the product to obtain the milk lactone perfume, wherein the aldol reaction comprises: uniformly mixing part of cyclohexanone serving as a base material with the alkaline water, heating the mixture, dropwise adding a mixture of the remaining cyclohexanone and n-butyraldehyde, stirring the materials while the mixture is dropwise added, continuously stirring after the dropwise adding is ended, and ending the heat-preserved reaction till the content of the n-butyraldehyde is less than 1 percent; allowing the solution to stand still, separating an oil layer, collecting a water layer for recycling and use; washing the collected oil layer with water, then transferring the oil layer into a distillation still for distillation to recycle excessive cyclohexanone for use; collecting a condensation product at the bottom of the distillation still. According to the continuous synthesis method, the yield of the aldol reaction is increased by adding the cyclohexanone in different ways; furthermore, under a reaction temperature condition of the continuous synthesis method, an aldol condensation product can be obtained in one step.
Owner:安徽华业香料合肥有限公司
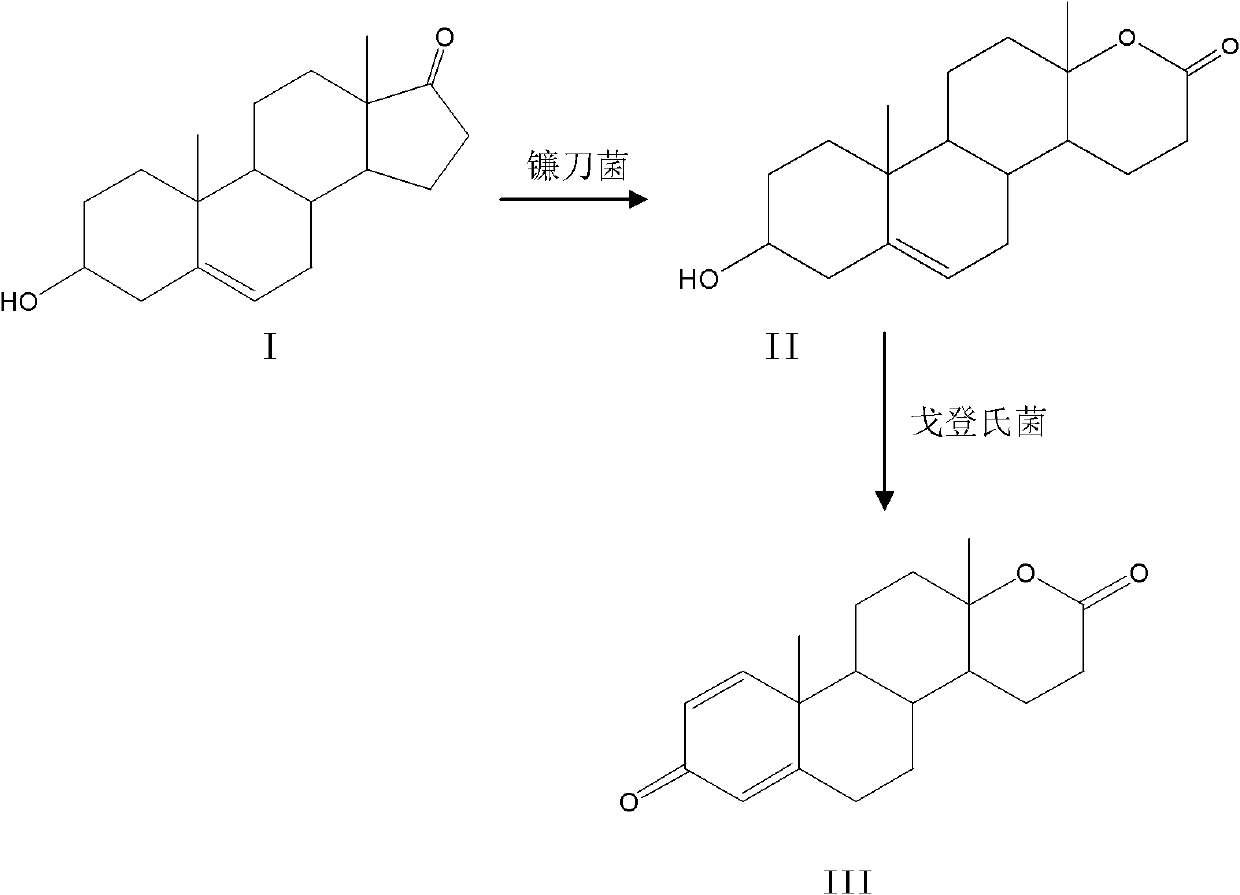
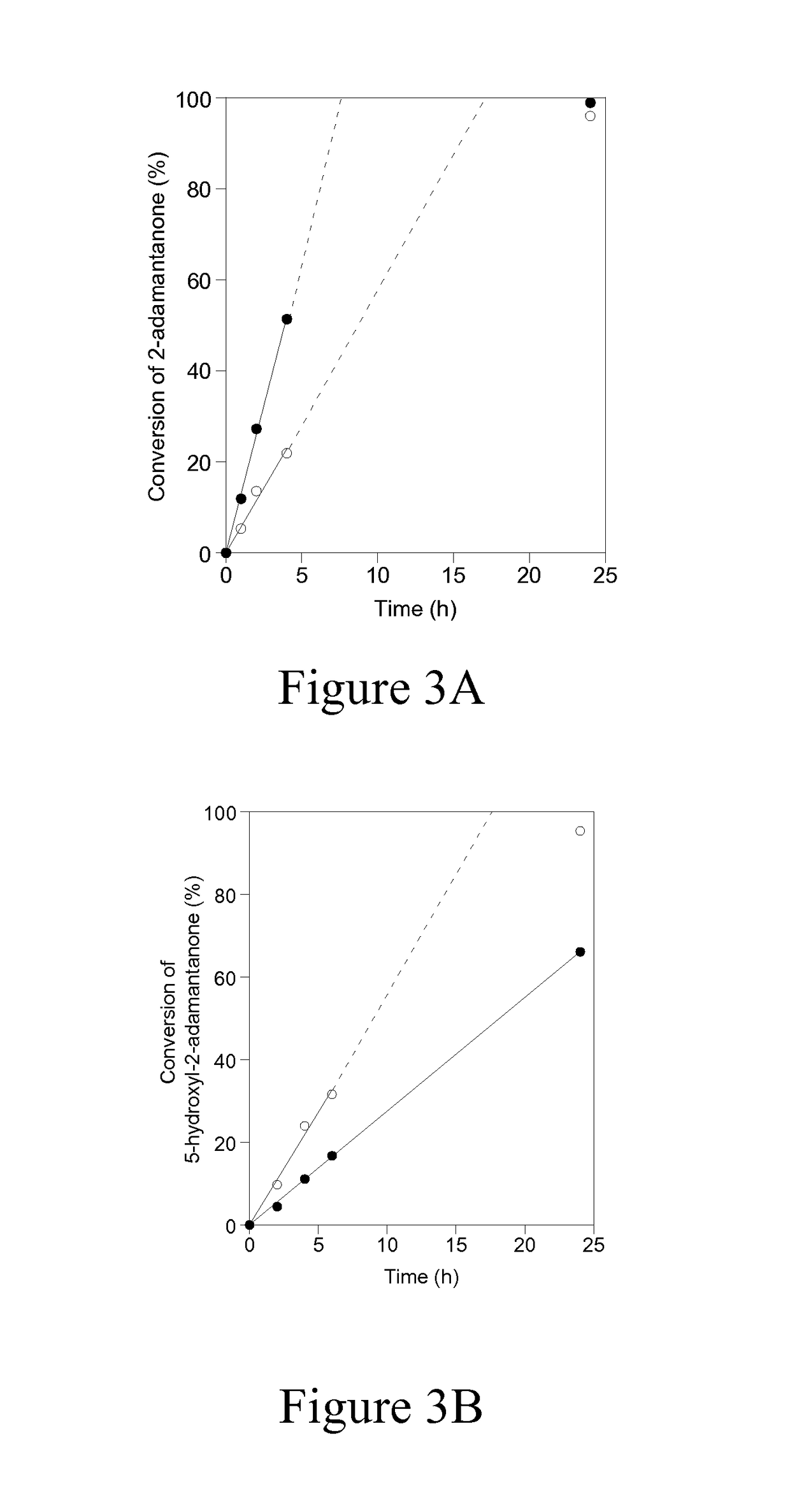
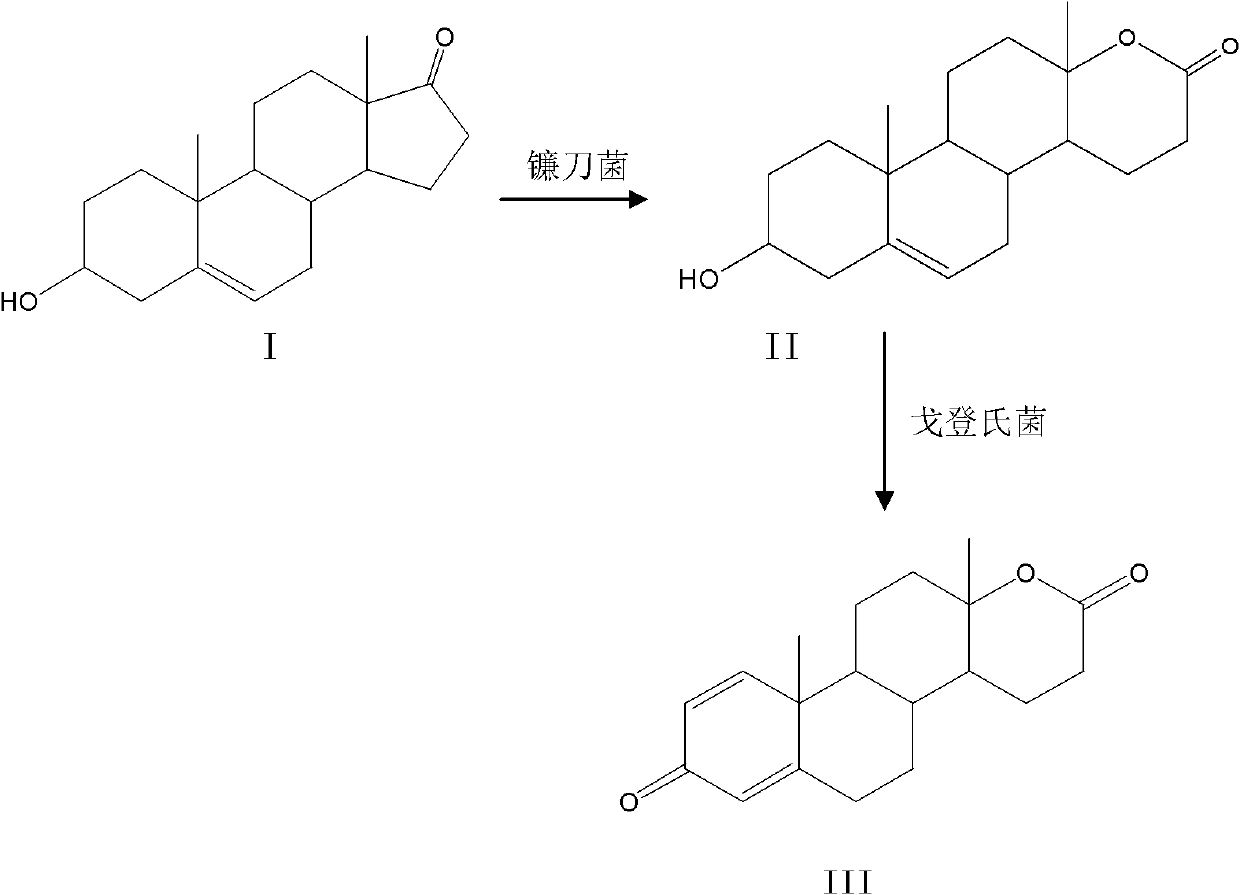
Method for preparing testolactone by microbial transformation
InactiveCN102703560ALow priceOptimize the process routeMicroorganism based processesFermentationMicroorganismBaeyer–Villiger oxidation
The invention discloses a method for preparing testolactone by microbial transformation, which mainly comprises the following steps: dissolving dehydroepiandrosterone in a solubilizer, adding the mixture in a transformation solution to prepare a transformation substrate solution; adding the transformation substrate solution into a transformation reaction device, adding fusarium oxysporum, transforming to obtain an intermediate of 3beta-hydroxy-17alpha-oxo-D-hexocyclo-androst-5-ene-17-ketone under the fusarium oxysporum resting cell transformation action through a Baeyer-Villiger oxidation reaction, adding gordonia after the transformation reaction is completed, performing a transformation reaction of the intermediate under the gordonia resting cell action to obtain testolactone. The method for preparing testolactone of the invention has the advantages of simple process route, easily available raw materials, cheap auxiliary materials, high substrate transformation rate, few by products, low production cost, and the like, overcomes the deficiencies of original production technology, and is suitable for industrial production.
Owner:SICHUAN NORMAL UNIVERSITY
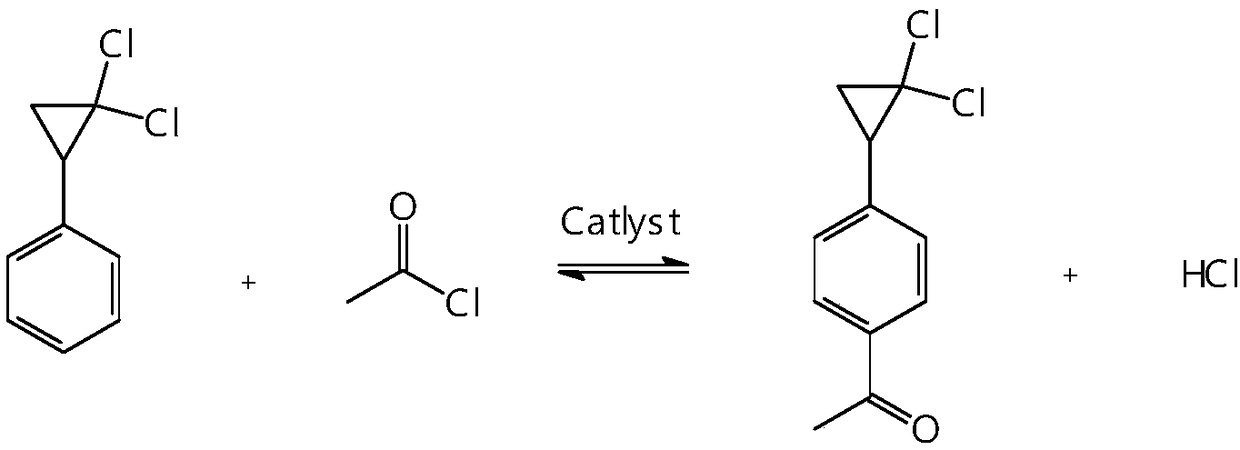
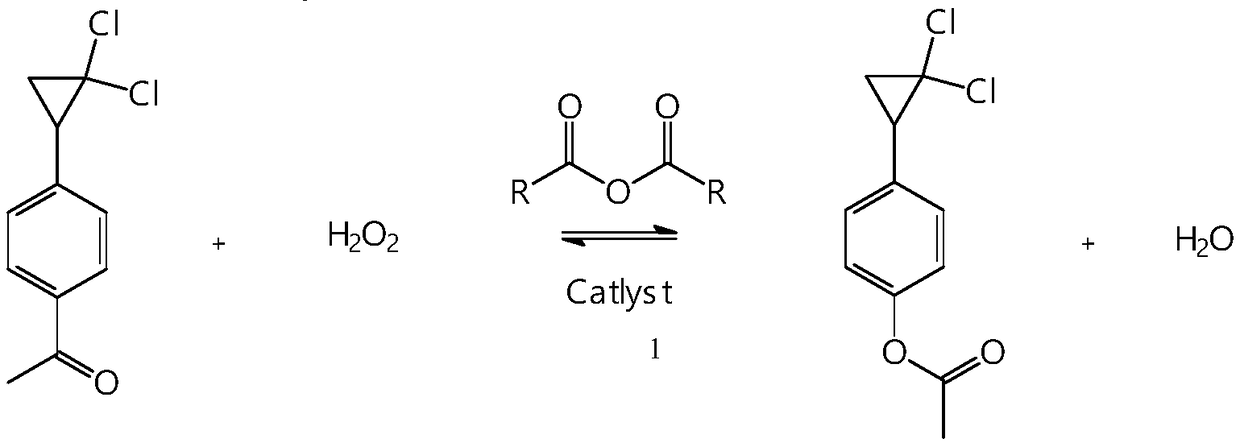
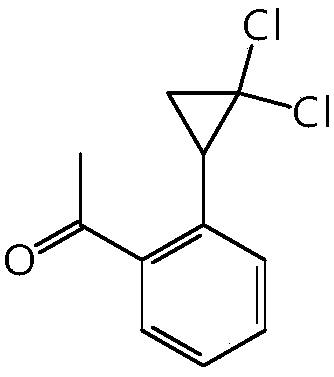
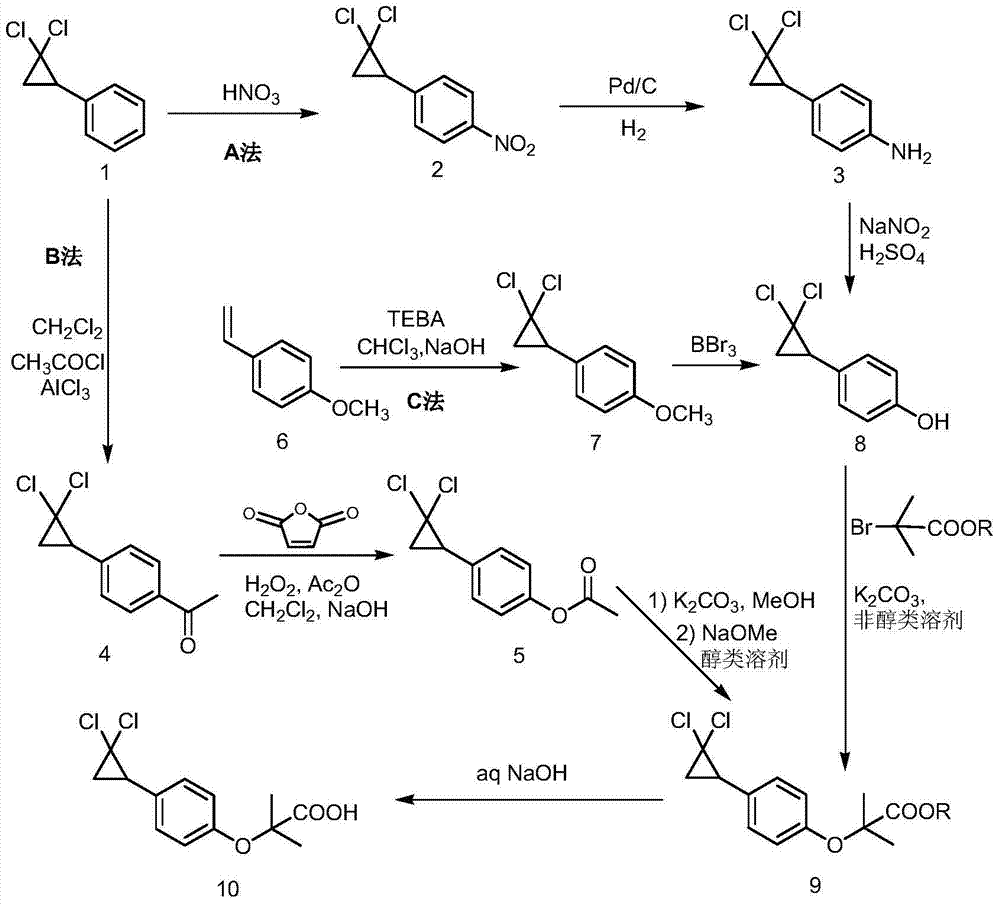
Synthetic method of 4-(2,2-dichloro cyclopropyl)phenol acetate
InactiveCN108658764ALow reaction temperatureHigh selectivityOrganic compound preparationCarboxylic acid esters preparationBaeyer–Villiger oxidationAcetic acid
The invention discloses a synthetic method of 4-(2,2-dichloro cyclopropyl)phenol acetate. The synthetic method comprises the following steps: taking 2,2-dichloro cyclopropyl benzene as a starting rawmaterial and carrying out electrophilic substitution reaction and baeyer-villiger oxidation reaction to synthesize the 4-(2,2-dichloro cyclopropyl)phenol acetate. According to the preparation method disclosed by the invention, an iodine element-containing co-catalyst is added into the electrophilic substitution reaction to reduce the temperature of the electrophilic substitution reaction, so thatthe selectivity of the electrophilic substitution reaction is improved; the content of an ortho-isomer is reduced within 0.3 percent by adopting relatively-inexpensive acetyl chloride as an electrophilic reagent; the activity of oxidation reaction is improved by adding an iron salt catalyst in the baeyer-villiger oxidation reaction, so that the reaction can be successfully carried out under the condition of taking acetic anhydride as an oxidizing medium, the yield of oxidation reaction is improved and potential safety hazards of a post-treatment process are reduced.
Owner:XINCHANG COUNTY TAIRU TECH CO LTD
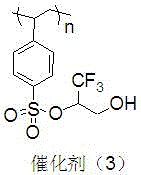
Immobilized fluorine-containing alcohol, method for preparing same and application of immobilized fluorine-containing alcohol
InactiveCN105665016ANovel structureEasy to manufactureOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsCross-linkSulfonyl chloride
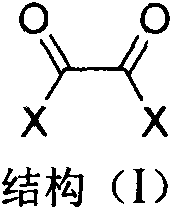
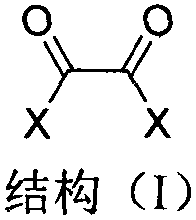
The invention discloses an immobilized fluorine-containing alcohol catalyst (shown as a formula I), a method for preparing the immobilized fluorine-containing alcohol catalyst and application thereof.An A in the formula I is independently selected from C2-C8 saturated multi-fluorine or total-fluorine carbon chains and C2-C8 unsaturated multi-fluorine or total-fluorine carbon chains.The method for preparing the immobilized fluorine-containing alcohol catalyst shown as the formula I includes steps of a, carrying out reaction on styrene-divinylbenzene cross-linked resin and sulfonyl chloride to obtain immobilized sulfonyl chloride (shown as a formula II); b, carrying out reaction on the immobilized sulfonyl chloride (shown as the formula II) and trimethyl silica fluorine-containing alcohol (shown as a formula III) to obtain sulfonate (shown as a formula IV) with immobilized trimethyl silica fluorine-containing alcohol; c, carrying out desilylation on the sulfonate (shown as the formula IV) with the immobilized trimethyl silica fluorine-containing alcohol under the effect of tetrabutylammonium fluoride to obtain the immobilized fluorine-containing alcohol catalyst (shown as the formula I).The immobilized fluorine-containing alcohol catalyst, the method and the application have the advantages that the immobilized fluorine-containing alcohol catalyst is easy to prepare and can be recycled; the immobilized fluorine-containing alcohol catalyst can be applied to Baeyer-Villiger oxidation reaction, and products are high in yield.
Owner:SHIJIAZHUANG UNIVERSITY
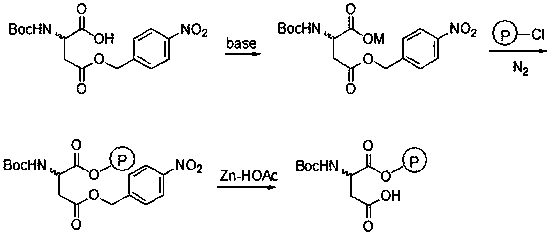
Baeyer-Villiger oxidation reaction catalyst and preparation method and application thereof
InactiveCN105498838ANovel structureThe synthetic route is simpleOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsBaeyer–Villiger oxidationTert-Butyloxycarbonyl protecting group
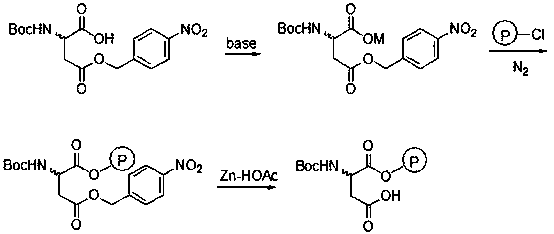
The invention discloses a Baeyer-Villiger oxidation reaction catalyst and a preparation method and application thereof. The Baeyer-Villiger oxidation reaction catalyst is N-t-butyloxycarboryl-aspartic acid immobilized by Merrifield resin, (S)-N-t-butyloxycarboryl-aspartic acid immobilized by Merrifield resin and (R)-N-t-butyloxycarboryl-aspartic acid immobilized by Merrifield resin. The preparation method of the catalyst comprises the following steps: 1, forming salt with N-Boc-aspartic acid-4-(4-nitro) benzyl ester and alkali; 2, making N-Boc-aspartic acid-4-(4-nitro) benzyl ester salt and chloromethyl groups on the Merrifield resin be subjected to a substitution reaction, so that N-Boc-aspartic acid-4-(4-nitro) benzyl ester immobilized by the Merrifield resin is obtained; 3, conducting debenzylation on the N-Boc-aspartic acid-4-(4-nitro) benzyl ester immobilized by the Merrifield resin in a Zn-HOAc system with the mass fraction being 90% at the temperature of 0 DEG C, so that the Baeyer-Villiger oxidation reaction catalyst is obtained. Peroxy acid can be generated by the catalyst and H2O2 under a certain condition in an in-situ mode, a Baeyer-Villiger oxidation reaction can be achieved safely and effectively, the product yield is high, a generated carboxylic acid by-product is the catalyst itself, catalyst consumption is little, and the catalyst can be utilized in a repeated and cyclic mode.
Owner:SHIJIAZHUANG UNIVERSITY
Method for manufacturing ester
InactiveUS8853426B2High catalytic activityHigh yieldOrganic oxidationOrganic compound preparationBaeyer–Villiger oxidationAryl
The present invention relates to a method for manufacturing an ester from a ketone or an aldehyde, which is a reactive substrate, by a Baeyer-Villiger oxidation reaction using hydrogen peroxide, and in this method, as a catalyst, M(BAr4)n, which is a metal borate, is used (M represents an alkali metal or an alkaline earth metal; Ar represents an aryl; and n is the same number as the valence of M). For example, when cyclohexanone was used as the reactive substrate, and Sr[B(3,5-CF3C6H3)4]2 was used as the catalyst, ε-caprolactone was obtained at an isolated yield of 82%.
Owner:NAGOYA UNIVERSITY
Industrialized preparation method of 1,4,5,8-naphthalene tetracarboxylic acid
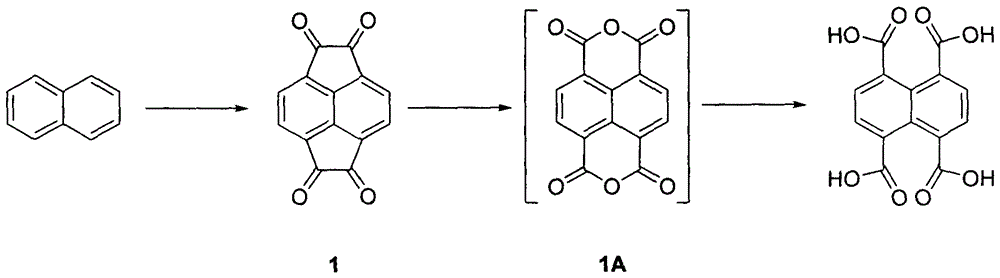
InactiveCN106146283AShort stepsImprove responsePreparation from carboxylic acid anhydridesCarbonyl compound preparation by condensationBaeyer–Villiger oxidationPurification methods
The invention is an industrialized preparation method of 1,4,5,8-naphthalene tetracarboxylic acid. The invention uses cheap and easy-to-obtain naphthalene as a starting material, and undergoes Friedel-Crafts acylation reaction, Baeyer-Villiger oxidation-hydrolysis "one 1,4,5,8-naphthalene tetracarboxylic acid is obtained after the pot method "reaction. Wherein the Friedel-Crafts acylation reaction yield is 87%, the Baeyer-Villiger oxidation-hydrolysis yield is 91%, and the total reaction yield is 79%. The present invention emphasizes the characteristics of short steps, simple reaction, high yield, simple purification method, easy operation and the like from the chemical point of view.
Owner:朱婧瑶
Method for preparing epsilon-caprolactone by using in-situ peroxide
The invention discloses a method for preparing epsilon-caprolactone by using in-situ peroxide. The method efficiently utilizes an in-situ peroxide obtained in a process of oxidizing alcohol by oxygento oxidize cyclohexanone into epsilon-caprolactone, i.e., under the catalysis of a catalyst, an alcohol is oxidized into the corresponding ketone while substances such as peroxy hydroxyl or hydrogen peroxide and the like generated in the process are fully utilized, so that the Baeyer Villiger oxidation reaction from cyclohexanone to epsilon-caprolactone is realized. Compared with a previous epsilon-caprolactone synthesis method, the method of the invention has the advantages that the product yield is remarkably increased, the use efficiency of alcohol is further improved, raw materials and reagents are cheap and easy to obtain, operation is easy, reaction conditions are mild, and the method is clean and environmentally friendly.
Owner:ZHEJIANG UNIV +2
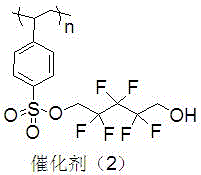
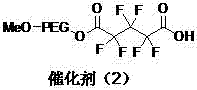
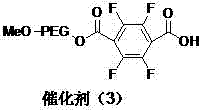
A kind of polyethylene glycol monomethyl ether immobilized fluorine-containing carboxylic acid catalyst and its preparation method and application
InactiveCN105521824BNovel structureEasy to manufactureOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPolyethylene glycolCarboxylic acid
The invention discloses a polyethylene glycol monomethyl ether immobilized fluorine-containing carboxylic acid catalyst (formula I), a preparation method and application thereof. In formula I, A is independently a C2-C12 saturated polyfluorinated or perfluorocarbon chain, a C2-C12 unsaturated polyfluorinated or perfluorocarbon chain, C6H3F, C6H2F2, C6HF3, C6F4. The preparation method of formula I comprises the following steps: a. fluorine-containing dicarboxylic acid monobenzyl ester (formula II) reacts with SOCl2 to prepare fluorine-containing dicarboxylic acid monobenzyl chloride (formula III); b. in the presence of alkali, Fluorine-containing dicarboxylic acid monobenzyl chloride (formula III) reacts with polyethylene glycol monomethyl ether to prepare polyethylene glycol monomethyl ether-supported fluorine-containing carboxylic acid ester (formula IV); c. polyethylene glycol The monomethyl ether-supported fluorine-containing carboxylic acid ester (formula IV) is hydrodebenzylated under the action of Pd-C to prepare polyethylene glycol monomethyl ether-immobilized fluorine-containing carboxylic acid catalyst (formula I). The catalyst is easy to prepare, the reaction catalyzed is a homogeneous reaction, the aftertreatment is simple, and the catalyst is easy to recover and recycle. The catalyst can be applied to Baeyer-Villiger oxidation reaction, the condition is mild, and the product yield is high.
Owner:SHIJIAZHUANG UNIVERSITY
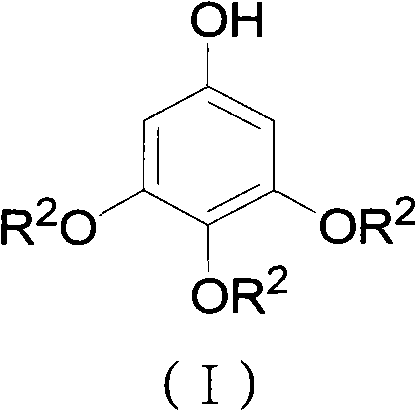
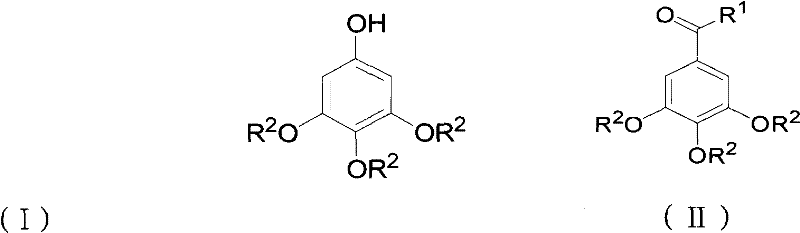
Preparation method for 3,4,5-trialkoxy phenol
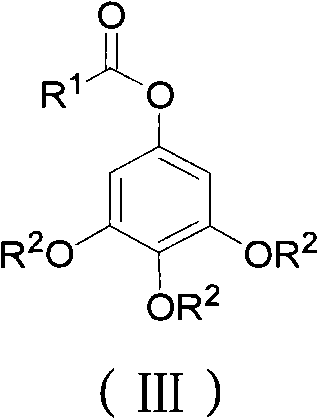
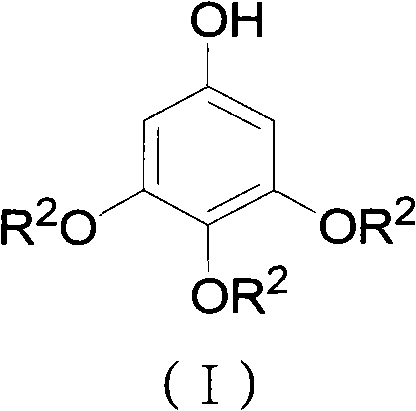
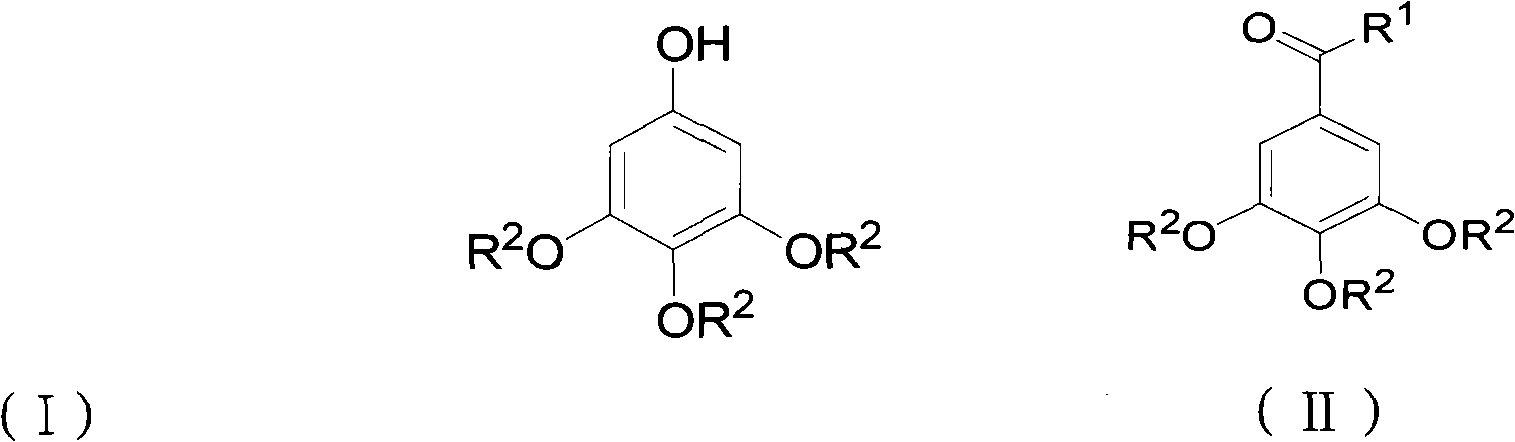
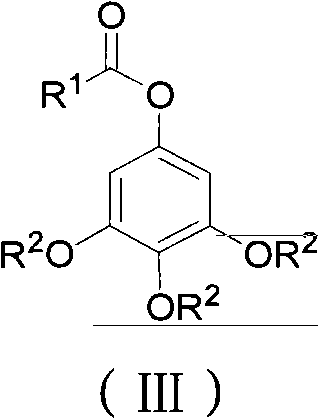
ActiveCN102452910AAvoid it happening againThe process steps are simpleOrganic chemistryOrganic compound preparationChemical synthesisBenzaldehyde
The invention relates to the field of chemical synthesis and discloses a preparation method for 3,4,5-trialkoxy phenol. According to the method, 3,4,5-trialkoxy benzaldehyde or 3,4,5-trialkoxy phenyl alkyl ketone and hydrogen peroxide are used as raw materials and are subjected to Baeyer-Villiger oxidation rearrangement reaction in a mixed solvent of dimethyl carbonate and methanol under the catalysis of a strong acid and then to basic hydrolysis, and 3,4,5-trialkoxy phenol is obtained after the strong acid is neutralized. The method provided in the invention has simple process steps, a yield of more than 50% and a remarkable application value in industrial production.
Owner:KPC PHARM INC
Preparation method for 3,4,5-trialkoxy phenol
ActiveCN102452910BAvoid it happening againThe process steps are simpleOrganic chemistryOrganic compound preparationChemical synthesisBenzaldehyde
The invention relates to the field of chemical synthesis and discloses a preparation method for 3,4,5-trialkoxy phenol. According to the method, 3,4,5-trialkoxy benzaldehyde or 3,4,5-trialkoxy phenyl alkyl ketone and hydrogen peroxide are used as raw materials and are subjected to Baeyer-Villiger oxidation rearrangement reaction in a mixed solvent of dimethyl carbonate and methanol under the catalysis of a strong acid and then to basic hydrolysis, and 3,4,5-trialkoxy phenol is obtained after the strong acid is neutralized. The method provided in the invention has simple process steps, a yield of more than 50% and a remarkable application value in industrial production.
Owner:KPC PHARM INC
Metal-catalyzed coupling of aryl and vinyl halides with alpha, alpha-difluorocarbonyl compounds
InactiveUS20160052854A1Carbamic acid derivatives preparationCarboxylic acid esters preparationArylBaeyer–Villiger oxidation
The coupling of aryl, heteroaryl, and vinyl halides with α,α-difluoroketones or silyl ethers or siylenol ethers of α,α-difluoroketones and α,α-difluoroamides and esters are described. Further derivatization of the coupling products (such as ketone cleavage and Baeyer-Villiger oxidation) is also described.
Owner:RGT UNIV OF CALIFORNIA
ε-n-pentyl propionate-ε-caprolactone and its preparation method
ActiveCN105001192BSignificant technological progressOrganic chemistryPropionateBaeyer–Villiger oxidation
The invention discloses an elpsilon-lactone perfume compound with a structure as described in the specification. The invention also discloses a preparation method for the elpsilon-lactone perfume compound. The preparation method comprises the following steps: with an acid compound and an alcohol compound as raw materials, subjecting the raw materials to esterification in an organic solvent under the action of an acid catalyst so as to obtain an amyl propionate compound; and with the amyl propionate compound and an alkene compound as raw materials carrying out addition and Baeyer-Villiger oxidation reaction under the action of an alkali catalyst and an acid catalyst so as to eventually obtain an elpsilon-amyl propionate-elpsilon-caprolactone compound. The novel elpsilon-lactone perfume compound with characteristic bouquet and fragrance of mellow and sweet rice wine is a novel research achievement of domestic and overseas research on elpsilon-lactone perfume compounds with characteristic fragrance.
Owner:CHINA GATEWAY PHARMA DEV CO LTD
A kind of immobilized fluorine-containing alcohol and its preparation method and application
InactiveCN105665016BNovel structureEasy to manufactureOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsSulfonyl chlorideCross-link
The invention discloses a solid-supported fluorine-containing alcohol catalyst (formula I), a preparation method and application thereof. In formula I, A is independently selected from C2-C8 saturated polyfluoro or perfluorocarbon chains, C2-C8 unsaturated polyfluoro or perfluorocarbon chains. The preparation method of formula I comprises the following steps: a. styrene-divinylbenzene cross-linked resin reacts with sulfonyl chloride to obtain immobilized sulfonyl chloride (formula II); b. under alkaline conditions, immobilized sulfonyl chloride (formula II ) is reacted with trimethylsiloxy fluorine-containing alcohol (formula III), and the sulfonate (formula IV) of immobilized trimethylsiloxy fluorine-containing alcohol is obtained; c. the sulfonic acid of immobilized trimethylsiloxy fluorine-containing alcohol The acid ester (formula IV) is desiliconized under the action of tetrabutylammonium fluoride to obtain a solid-supported fluorine-containing alcohol catalyst (formula I). The catalyst is easy to prepare and can be recycled. The catalyst can be applied to Baeyer-Villiger oxidation reaction, and the product yield is high.
Owner:SHIJIAZHUANG UNIVERSITY
Method for synthesizing delta-dodecalactone
InactiveCN102382089BHigh yieldHigh purityOrganic chemistryChemical recyclingBaeyer–Villiger oxidationDistillation
The present invention discloses a method for synthesizing delta-dodecalactone, which comprises the following steps: (1) condensating and dehydrating: under the present of a phase-transfer catalyst under an alkaline condition, causing aldol condensation between cyclopentanone and heptanal, and dehydrating for generating 2-heptene cyclopentanone under the function of an acid catalyst; (2) hydrogenating: causing the 2-heptene cyclopentanone to hydrogenate under the presence of ion exchange resin for obtaining 2- heptyl cyclopentanone; (3) oxidizing: causing a Baeyer-Villiger oxidation reaction between the 2- heptyl cyclopentanone and hydrogen peroxide for obtaining a crude product; and (4) refining: obtaining a pure product from the crude product through molecular distillation. The method for synthesizing delta-dodecalactone has the following advantages: easy method application, simple operation, higher yield, and easy available raw material. The yield of the target product delta-dodecalactone is improved, and the purity is greatly improved. The product purity is larger than 99.0%, and the yield is above 80%. The catalyst used in the method has the characteristics such as capability of being used repeatedly.
Owner:ANHUI UNIV OF SCI & TECH
Highly active, selective, accessible, and robust zeolitic Sn-baeyer-villiger oxidation catalyst
Provided is a process of conducting a Baeyer-Villiger oxidation which comprises contacting a ketone and an oxidant in the presence of an Sn-DZ-1 catalyst to thereby oxidize the ketone to an ester. The Sn-DZ-1 catalyst comprises Sn heteroatoms on the external surface of the zeolitic material lattice framework, and B heteroatoms, or silanols created from boron hydrolysis, throughout the remainder of the lattice framework.
Owner:RGT UNIV OF CALIFORNIA +1
Method for preparing testolactone by microbial transformation
InactiveCN102703560BLow priceOptimize the process routeMicroorganism based processesFermentationBaeyer–Villiger oxidationFusarium oxysporum
The invention discloses a method for preparing testolactone by microbial transformation, which mainly comprises the following steps: dissolving dehydroepiandrosterone in a solubilizer, adding the mixture in a transformation solution to prepare a transformation substrate solution; adding the transformation substrate solution into a transformation reaction device, adding fusarium oxysporum, transforming to obtain an intermediate of 3beta-hydroxy-17alpha-oxo-D-hexocyclo-androst-5-ene-17-ketone under the fusarium oxysporum resting cell transformation action through a Baeyer-Villiger oxidation reaction, adding gordonia after the transformation reaction is completed, performing a transformation reaction of the intermediate under the gordonia resting cell action to obtain testolactone. The method for preparing testolactone of the invention has the advantages of simple process route, easily available raw materials, cheap auxiliary materials, high substrate transformation rate, few by products, low production cost, and the like, overcomes the deficiencies of original production technology, and is suitable for industrial production.
Owner:SICHUAN NORMAL UNIV
A kind of ε-lactone type fragrance compound and preparation method thereof
Owner:CHINA GATEWAY PHARMA DEV CO LTD
Solid acid catalyst, preparation method and application
InactiveCN104588049BEasy to prepareSuitable for industrial mass productionOrganic chemistryPhysical/chemical process catalystsIndiumCerium
Owner:NANJING UNIV OF INFORMATION SCI & TECH
The synthetic method of ciprofibrate
ActiveCN103613498BReduce reactivityHigh yieldOxygen-containing compound preparationOrganic compound preparationAcetic anhydrideOrtho position
The invention discloses a synthetic method of ciprofibrate. The method comprises the following steps: taking styrene as a starting material; obtaining the ciprofibrate by the processes of cyclization, acylation, Baeyer-Villiger oxidation, alcoholysis, alkylation and hydrolysis, wherein an acylation reagent adopted in the acylation process is R1COCl; the R1 is C4-C12 alkyl. Fatty acyl chloride of which a hydrocarbyl structure is a long chain is adopted in acylation reaction, so that the steric hindrance is increased, the reaction activity of acyl chloride is reduced, the content of the produced ortho-position isomer is smaller than 0.2% and superior to that of acetyl chloride reaction (the content of the ortho-position isomer is 0.5-1%), the yield and the purity of the product are improved, and meanwhile, a room-temperature reaction condition is adopted, so that the energy consumption of production is reduced. Urea peroxide instead of hydrogen peroxide is used as an oxidant in the Baeyer-Villiger oxidation, and acetic acid is used instead of acetic anhydride, so that the reaction condition is milder, and the operation is more controllable.
Owner:ZHEJIANG SANMEN HYGECON PHARMA CO LTD
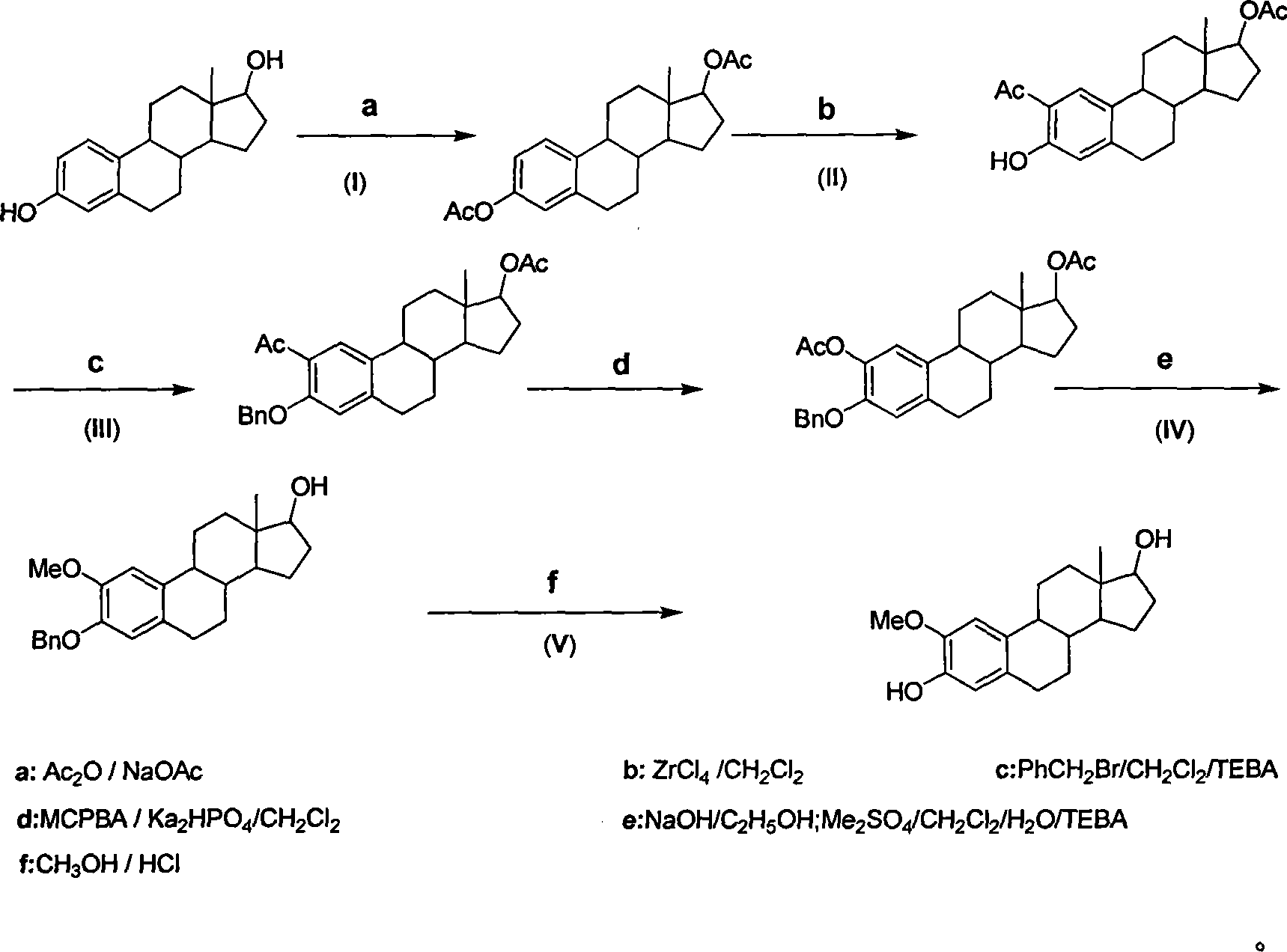
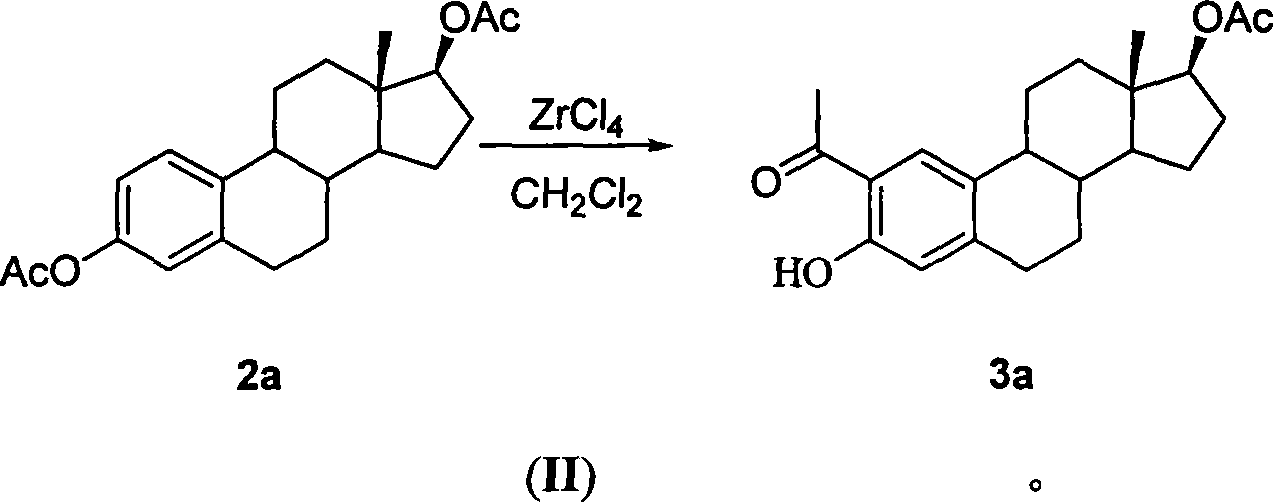
Method for synthesizing 2-methoxy estradiol
InactiveCN101161669AEfficient separationEasy to observe whether the reaction is complete or notSteroidsAcetic anhydrideHydrolysis
The present invention belongs to the field of organic chemistry and drug synthesis, and relates to a synthetic method of 2-methoxyestradiol. The present invention adopts estradiol as the original raw material, which undergoes six reactions including acylation with acetic anhydride and acetate as the reagents, and a mixture of petroleum benzene and acetone with the ratio of 20:1 which is used as the TLC developer to trace the recomposition, a phase-transference benzyl ether protection, Baeyer-Villiger oxidation, one-step 2-,17-diacetoxylide hydrolysis -2-methylation, concentrated and hydrochloride acid 3- protection to product the intended product of 2-methoxyestradiol. The present invention has mild reaction condition, simple operation, the reagent which is easily accessible with low cost, thorough reaction, shortened reaction duration, simple post treatment, raised recovery rate and raised purity of the product, economized reagent and the decreased cost, as well as the prospect of industrialized production.
Owner:FUDAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com