Synthesis method of 1-pyrenol and intermediates thereof
A synthesis method and a technology for hydroxypyrene are applied in chemical instruments and methods, preparation of carbonyl compounds by condensation, preparation of organic compounds, etc., and can solve problems such as difficulty in demethylation, difficulty in industrialization, high price of 1-bromopyrene, and the like, To achieve the effect of easy operation, saving production cost and less pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1, the synthetic method of a kind of 1-hydroxypyrene of the present invention and its intermediate comprises the following steps:
[0032] Step 1, utilizing the Friedel-Crafts acylation reaction of pyrene and acetyl chloride to prepare the intermediate acetylpyrene;
[0033] Step 2, using the bayer-villiger oxidative rearrangement reaction (Baeyer-Villiger oxidation rearrangement reaction, Bayer-Villiger oxidative rearrangement reaction is an oxidation reaction in which peroxyacid oxidizes ketones into esters, and occurs in the reaction Nucleophilic rearrangement, commonly used peroxyacids in the reaction are peroxyacetic acid, peroxytrifluoroacetic acid, peroxybenzoic acid, 3-chloroperoxybenzoic acid, peroxysulfuric acid, etc.), to prepare the intermediate acetoxypyrene ;
[0034] Step 3, preparing 1-hydroxypyrene by saponification reaction.
[0035] The detailed steps are as follows:
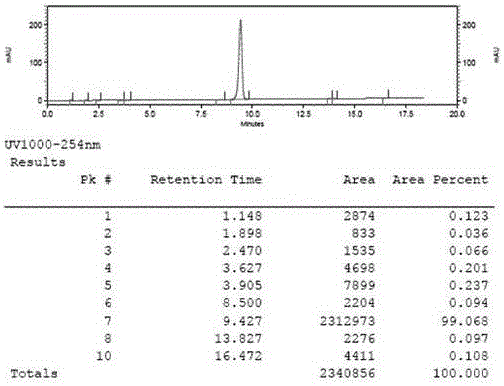
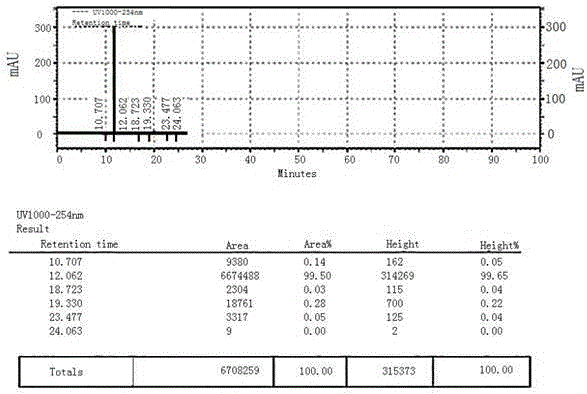
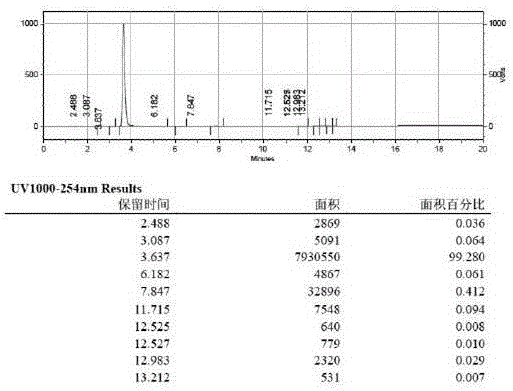
[0036] Synthesis of intermediate 1-acetylpyrene, add 202g of pyrene (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com