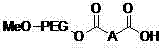A kind of polyethylene glycol monomethyl ether immobilized fluorine-containing carboxylic acid catalyst and its preparation method and application
A technology of polyethylene glycol monomethyl ether and fluorine-containing carboxylic acid, which is applied to the preparation of carboxylate esters, chemical instruments and methods, and the preparation of organic compounds, and can solve the problems of unsuitable industrial production, troublesome recycling, and the price of pentafluorobenzoic acid. Expensive and other issues, to achieve the effect of novel structure and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of polyethylene glycol monomethyl ether immobilized fluorine-containing carboxylic acid catalyst (1).
[0025] The structure of the catalyst (1) is shown in the following formula:
[0026]
[0027] 2.80 g (0.01 moL) of 2,2,3,3-tetrafluoro-1,4-butanedioic acid monobenzyl ester was added to 11.8 g of SOCl 2 Add 5 drops of DMF, heat to reflux for 2h, evaporate SOCl under reduced pressure 2 , to obtain 2,2,3,3-tetrafluoro-1,4-butanedioic acid monobenzyl chloride.
[0028] N 2 Under protection, 30g (0.005moL) MeO-PEG-OH 6000 was dissolved in 50mL CH 2 Cl 2 In ice bath cooling, add 1.21g (0.012moL) Et 3 N, stirring, slowly drop 2.98g (0.01moL) 2,2,3,3-tetrafluoro-1,4-butanedioic acid monobenzyl chloride and 5mL CH 2 Cl 2 After the dropwise addition, it was naturally raised to room temperature, reacted for 24 hours, and evaporated 30mLCH 2 Cl 2 , under stirring, the remaining reaction solution was added to 200mL of anhydrous ether, settled for 30min, fi...
Embodiment 2
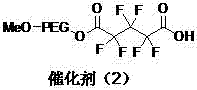
[0032] Preparation of polyethylene glycol monomethyl ether immobilized fluorine-containing carboxylic acid catalyst (2).
[0033] The structure of the catalyst (2) is shown in the following formula:
[0034]
[0035] Replace 2.80g (0.01moL) of 2,2,3,3-tetrafluoro- 1,4-Monobenzyl succinate, and other operations were the same as in Example 1 to prepare catalyst (2). The amount of carboxyl groups immobilized in the catalyst (2) is 0.72mmol / g.
Embodiment 3
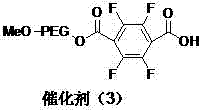
[0037] Preparation of polyethylene glycol monomethyl ether immobilized fluorine-containing carboxylic acid catalyst (3).
[0038] The structure of the catalyst (3) is shown in the following formula:
[0039]
[0040] Replace 2.80 g (0.01 moL) of 2,2,3,3-tetrafluoro-1,4-butanedioic acid monobenzyl ester with 3.28 g (0.01 moL) of 2,3,5,6-tetrafluoroterephthalic acid monobenzyl ester Benzyl ester, replace 1.21g (0.012moL) Et with 0.95g (0.012moL) pyridine 3 N, other operations were the same as in Example 1 to prepare catalyst (3). The amount of carboxyl groups immobilized in the catalyst (3) is 0.70mmol / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com