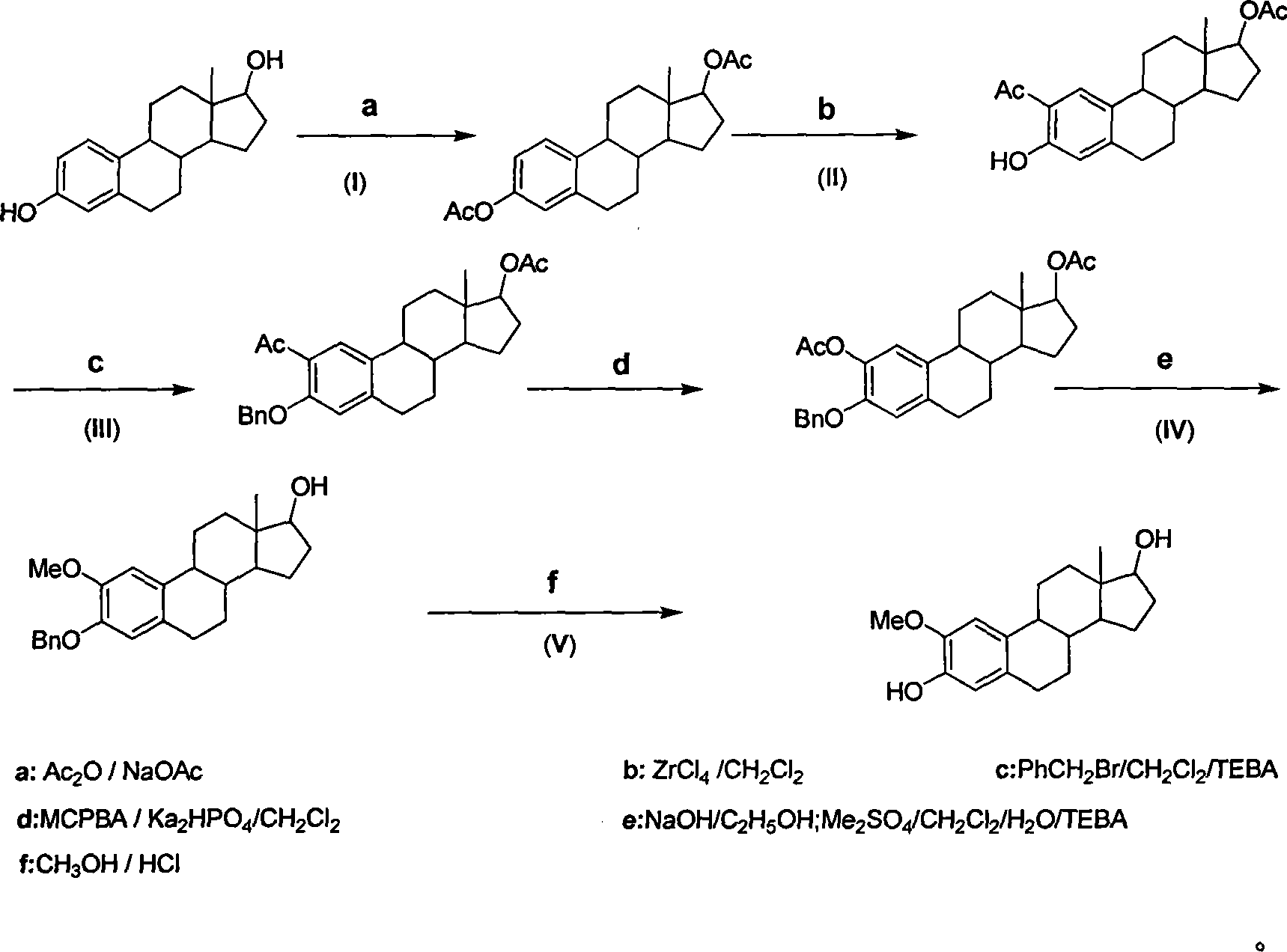
Method for synthesizing 2-methoxy estradiol
A technology of methoxyestradiol and a synthesis method, which is applied in the field of synthesizing compound 2-methoxyestradiol, can solve the problem of difficulty in distinguishing between substrate and product spots, affecting the quality reaction of intermediates, difficult recycling of DMF, etc. problems, to achieve the effect of saving reagents, simplifying operation, and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
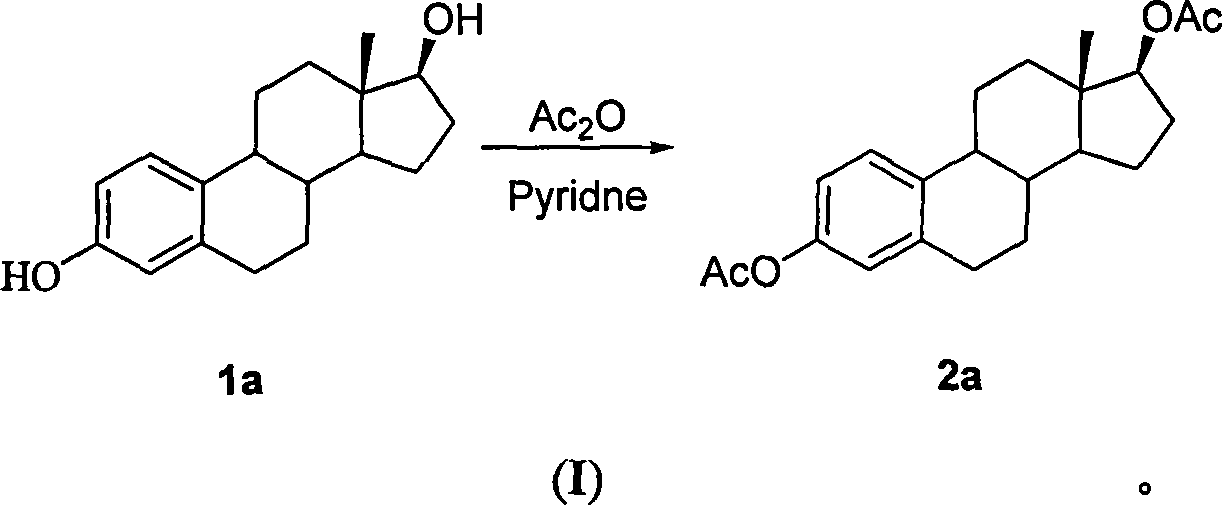
[0031] Example 1: 1,3,5(10)-triene-3,17β-estradiol diethyl ester
[0032] Add 500mg (1.84mmol) of estradiol and 1.51g (18.36mmol) NaOAc to 10.35mL (110.14mmol) of Ac 2 O, heated to reflux. The initial reaction solution was a white suspension, which was basically dissolved after heating for five minutes, and a large amount of white foamy substances were produced. Heating at reflux for 30min was complete. The acetic anhydride was removed under reduced pressure and washed with CHCl 3 Extraction reaction liquid, saturated Na 2 CO 3 (aq.) Wash twice, then wash with water until neutral. Combined aqueous phase, CHCl 3 Reverse phase extraction twice. The organic phases were combined and the solvent was removed to give 830 mg of a white solid. Hot methanol recrystallization gave 649.75mg of white scaly crystals, mp: 120-122°C, yield 99.14%. Ms (m / z, 100%): 356 (M, 8.91), 314 (basepeak, 100%)
[0033] 1 H-NMR (CDCl 3 ): δ0.83(s, 3H, C 18 -H), 2.05(s, 3H, C 17 -OCOCH 3 ), 2....
Embodiment 2
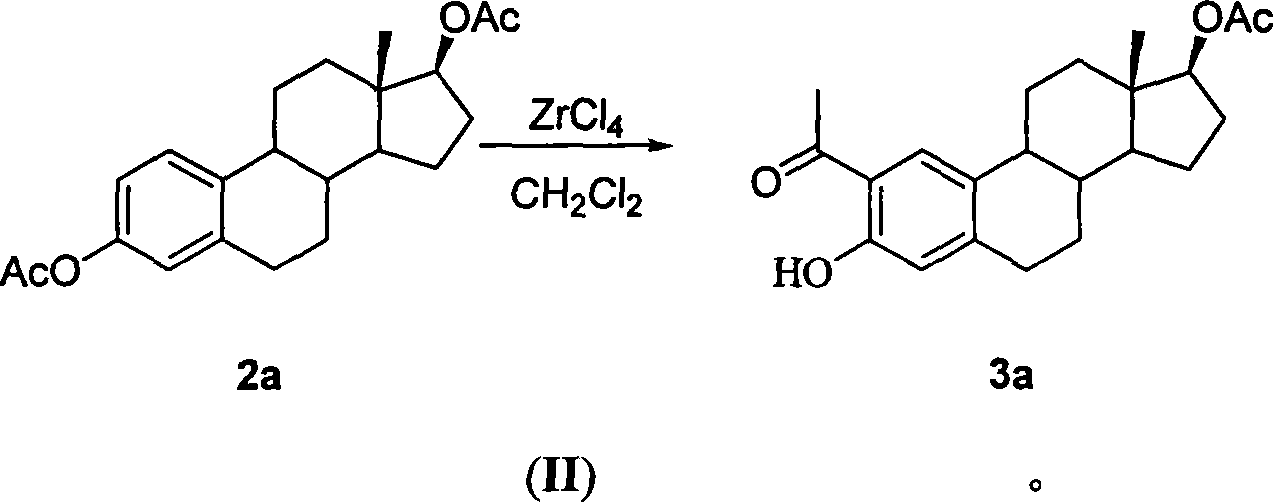
[0034] Example 2: 2-acetyl-3 benzyloxy-1,3,5(10)-triene-17β-dihydroxy-17-acetate Weigh 4.21g (11.8mmol) 2-acetyl-1 , 3,5(10)-triene-3,17β-alcohol acetate was dissolved in 108mL CH 2 Cl 2 Turn into yellow clear liquid, add 2.69g (11.80mmol) TEBA, dissolve completely. Weigh 16.87g of NaOH to prepare a 20% NaOH solution and add it to the reaction solution. Then measure 4.22mL (35.40mmol) PhCH 2 Br was added to the reaction solution. The reaction solution was separated into layers and stirred vigorously at room temperature for 72 hours. TLC (benzene:acetone 30:2) showed that the reaction was complete. Add 100 mL of ice water, and continue stirring for 1 hour in the ice water bath. use CH 2 Cl 2 Extract three times, combine the organic phase and wash three times with water. The crude weight of the dried solvent was 5.82 g. Recrystallization from hot methanol gave 3.14g, mp: 159-160°C. Recover the mother liquor to obtain: 1.43g, mp: 158-160°C, and dry the mother liquor: 0...
Embodiment 3
[0037] Example 3: 2-methoxy-3-benzyloxy-1,3,5(10)-triene-17β-estradiol
[0038] Weigh 1.00g (2.16mmol) 3-benzyloxy-1,3,5(10)-triene-2,17β-diol diacetate, dissolve in 68mL C 2 h 5 OH, into a white suspension. Add 224.72mg (5.62mmol) of NaOH, and the reaction liquid becomes clear to light red rapidly after heating. Heating to reflux for 1h, TLC showed that the reaction was complete. Ethanol was removed under reduced pressure. Add 60mL CH directly to the reaction solution without separation 2 Cl 2 , 50mL H 2 O, 492.68mg (2.16mmol) TEBA, 1.00mL dimethyl sulfate, add 2.50g NaOH, the measured pH=14. After 4 hours of reaction, TLC (benzene:acetone 8:2) showed that the reaction was complete. Wash to neutral, CH 2 Cl 2 Extract twice, combine the organic phases, and pull dry crude weight 1.43g. 0.75 g of 7a was obtained by using petroleum ether: ethyl acetate 20:4 as developing solvent to obtain 0.75 g of 7a, mp: 98°C-100°C. The yield was 88.06%. Ethyl acetate was recrystal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com