Method for preparing epsilon-caprolactone by using in-situ peroxide
A technology of peroxide and cyclohexanone peroxide, which is applied in the chemical field, can solve the problems such as the decrease in the use efficiency of hydrogen peroxide, the unsatisfactory yield of ε-caprolactone, and the reduction of the selectivity of ε-caprolactone. , to achieve the effects of cheap catalyst, excellent selectivity, and increased reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
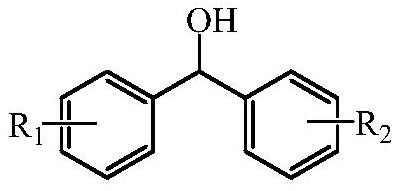
[0045] Add N-hydroxyphthalimide (NHPI, 75mg, 0.45mmol), azobisisobutyronitrile (AIBN, 38mg, 0.23mmol), diphenylmethanol (1104mg, 6mmol), cyclohexane Ketone (392mg, 4mmol) and ethyl acetate (AcOEt, 1.5mL) were gas exchanged (pure oxygen) three times, and reacted at 75°C for 8 hours. Lower the temperature of the reaction solution to 30°C, add 1,2-bis(3,5-bis(trifluoromethyl)phenyl)diselenide (Se-Cat1, 70mg, 0.12mmol), trifluoroethanol (TFE, 15mL ), continue to react for 6 hours, and obtain 3a 99% (with biphenyl as internal standard, gas phase yield), 4 99% (with biphenyl as internal standard, gas phase yield). The ε-caprolactone product was separated, and the reaction mother liquor was distilled off under reduced pressure to remove the solvent, then 20 mL of ether was added to dissolve, and then extracted with water (10 mL×3), and the aqueous phase was dried by rotary evaporation to obtain 419 mg (92% )ε-caprolactone product with a purity of 97%.
[0046] 4 (ε-capr...
Embodiment 2
[0048]
[0049] Add N-hydroxyphthalimide (NHPI, 75mg, 0.45mmol), azobisisobutyronitrile (AIBN, 38mg, 0.23mmol), and 4,4'-dimethylbenzylmethanol to the three-necked flask (1272mg, 6 mmol), cyclohexanone (392mg, 4mmol) and ethyl acetate (AcOEt, 1.5mL), gas exchange (pure oxygen) three times, and react at 75°C for 8 hours. Lower the temperature of the reaction solution to 30°C, add 1,2-bis(3,5-bis(trifluoromethyl)phenyl)diselenide (Se-Cat1, 70mg, 0.12mmol), trifluoroethanol (TFE, 15mL ), continue to react for 6 hours to obtain 3b 99% (with biphenyl as internal standard, gas phase yield), 4 99% (with biphenyl as internal standard, gas phase yield).
Embodiment 3
[0051]
[0052] Add N-hydroxyphthalimide (NHPI, 75mg, 0.45mmol), azobisisobutyronitrile (AIBN, 38mg, 0.23mmol), 4,4'-di-n-propyldiphenyl Methanol (1608mg, 6mmol), cyclohexanone (392mg, 4mmol) and ethyl acetate (AcOEt, 1.5mL), gas exchanged (pure oxygen) three times, reacted at 75°C for 8 hours. Lower the temperature of the reaction solution to 30°C, add 1,2-bis(3,5-bis(trifluoromethyl)phenyl)diselenide (Se-Cat1, 70mg, 0.12mmol), trifluoroethanol (TFE, 15mL ), continue to react for 6 hours, obtain 3c 99% (with biphenyl as internal standard, gas phase yield), 4 99% (with biphenyl as internal standard, gas phase yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com