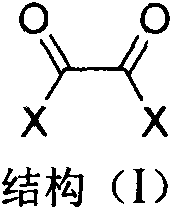
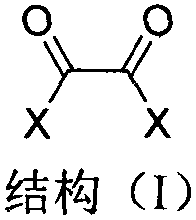
Industrialized preparation method of 1,4,5,8-naphthalene tetracarboxylic acid
A technology of naphthalenetetracarboxylic acid and acylation, applied in 1 field, can solve the problems of many three wastes, long steps, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] step one:
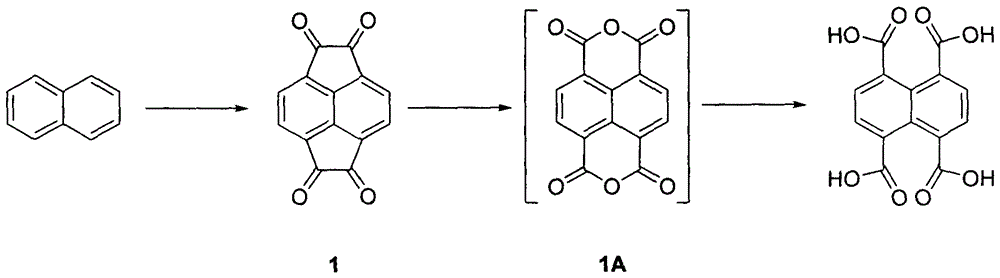
[0020] To naphthalene (40 g, 0.31 mol) in carbon disulfide (2 L) was added AlCl in portions at 0 °C 3 (206.6g, 1.55mol), then warmed up to 10°C and stirred for 20min. Oxalyl chloride (78.7 g, 0.62 mol) was slowly added dropwise to the solution, and after the dropwise addition was completed, it was raised to room temperature and stirred for 6 h. The temperature was lowered to 0°C, 150mL of concentrated hydrochloric acid and 2L of chloroform were added to the reaction solution, and the organic phase was washed with water, washed with saturated brine, and dried to obtain compound 1 (63.7g), with a yield of 87%. Proton NMR spectrum (400MHz, DMSO-d 6 )δ: 8.28 (4H, s), Calcd for C 14 h 4 o 4 : 236.0110, Found: 236.0102.
[0021] Step two:
[0022] After a mixed solution of compound 1 (50 g, 0.21 mol), NaOH (33.6 g, 0.84 mol) in 1,4-dioxane (240 mL) and water (90 mL) was warmed to 60 ° C, slowly added dropwise H 2 o 2 (35.7 mL, 30%, 0.315 mol) was added dr...
Embodiment 2
[0024] step one:
[0025] To naphthalene (40 g, 0.31 mol) in carbon disulfide (2 L) was added AlBr in portions at 0 °C 3 (413.4g, 1.55mol), then warmed up to 10°C and stirred for 20min. Oxalyl bromide (133.8 g, 0.62 mol) was slowly added dropwise to the solution, and after the dropwise addition was completed, it was raised to room temperature and stirred for 6 h. The temperature was lowered to 0°C, 150mL of concentrated hydrochloric acid and 2L of chloroform were added to the reaction solution, the organic phase was washed with water, washed with saturated brine, and dried to obtain compound 1 (58.5g), with a yield of 80%. Proton NMR spectrum (400MHz, DMSO-d 6 )δ: 8.28 (4H, s), Calcd for C 14 h 4 o 4 : 236.0110, Found: 236.0102.
[0026] Step two:
[0027] After a mixed solution of compound 1 (50 g, 0.21 mol), NaOH (33.6 g, 0.84 mol) in 1,4-dioxane (240 mL) and water (90 mL) was warmed to 60 ° C, slowly added dropwise H 2 o 2 (35.7 mL, 30%, 0.315 mol) was added dropwi...
Embodiment 3
[0029] step one:
[0030] To naphthalene (40 g, 0.31 mol) in carbon disulfide (2 L) was added AlCl in portions at 0 °C 3 (206.6g, 1.55mol), then warmed up to 10°C and stirred for 20min. Oxalyl chloride (78.7 g, 0.62 mol) was slowly added dropwise to the solution, and after the dropwise addition was completed, it was raised to room temperature and stirred for 6 h. The temperature was lowered to 0°C, 150mL of concentrated hydrochloric acid and 2L of chloroform were added to the reaction solution, and the organic phase was washed with water, washed with saturated brine, and dried to obtain compound 1 (63.7g), with a yield of 87%. Proton NMR spectrum (400MHz, DMSO-d 6 )δ: 8.28 (4H, s), Calcd for C 14 h4 o 4 : 236.0110, Found: 236.0102.
[0031] Step two:
[0032] To a mixed solution of compound 1 (50 g, 0.21 mol), NaOH (33.6 g, 0.84 mol) in 1,4-dioxane (240 mL) and water (90 mL) was added m-chloroperoxybenzene in portions at room temperature After the addition of formic aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com