Immobilized fluorine-containing alcohol, method for preparing same and application of immobilized fluorine-containing alcohol
A technology of fluorine-containing alcohols and perfluorocarbons, applied in chemical instruments and methods, catalytic reactions, organic compounds/hydrides/coordination complex catalysts, etc., can solve problems such as unsuitable for industrial production, large consumption, and troublesome recycling. Achieve the effect of novel structure, simple processing and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of polyethylene glycol monomethyl ether immobilized fluorine-containing alcohol catalyst (1).
[0022] The structure of the catalyst (1) is shown in the following formula.
[0023]
[0024] Synthesis of styrene-divinylbenzene crosslinking resin (2% divinylbenzene) immobilized sulfonyl chloride (formula II), prepared by reference method (J.Org.Chem.1979,44,4634) (Cl content is 4.73 mmol / g).
[0025] Add 0.42g (1mmolCl) of styrene-divinylbenzene crosslinking resin immobilized sulfonyl chloride to 1.88g (8mmol) of 2,2,3,3-tetrafluoro-4-trimethylsiloxy-1-butanol, 0.28 mL (2 mmol) Et 3 N with 30mLCH 2 Cl 2 In the mixture, react at room temperature for 24h, filter, methanol (15mL×2), H 2 O (15mL×2) and methanol (15mL×2) were washed and dried in vacuo to obtain 0.88g of 2,2,3,3-tetrafluoro-4-trimethylsilyloxy-1-butanolsulfonate on immobilized basis. Through the analysis of sulfur element content, the measured sulfur content is 7.392%, and its equivalent fl...
Embodiment 2
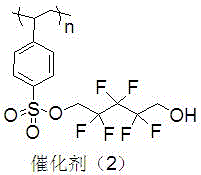
[0028] Preparation of polyethylene glycol monomethyl ether immobilized fluorine-containing alcohol catalyst (2).
[0029] The structure of the catalyst (2) is shown in the following formula.
[0030]
[0031] Replace 1.88g (8mmol) in Example 1 with 2.28g (8mmol) 2,2,3,3,4,4-hexafluoro-5-trimethylsiloxy-1-pentanol and 0.16g (2mmol) pyridine respectively 2,2,3,3-Tetrafluoro-4-trimethylsiloxy-1-butanol and 0.28 mL (2 mmol) Et 3 N, the other operations were the same, and 0.80 g of catalyst (2) was obtained, and the immobilized amount of fluoroalcohol was 2.29 mmol / g.
Embodiment 3
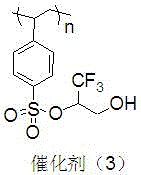
[0033] Preparation of polyethylene glycol monomethyl ether immobilized fluorine-containing alcohol catalyst (3).
[0034] The structure of the catalyst (3) is shown in the following formula.
[0035]
[0036] Replace the 2.28g (8mmol) 2,2,3,3,4,4-hexa Fluoro-5-trimethylsilyloxy-1-pentanol was operated in the same manner to obtain 0.62 g of catalyst (3), and the immobilized amount of fluoroalcohol was 2.15 mmol / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com