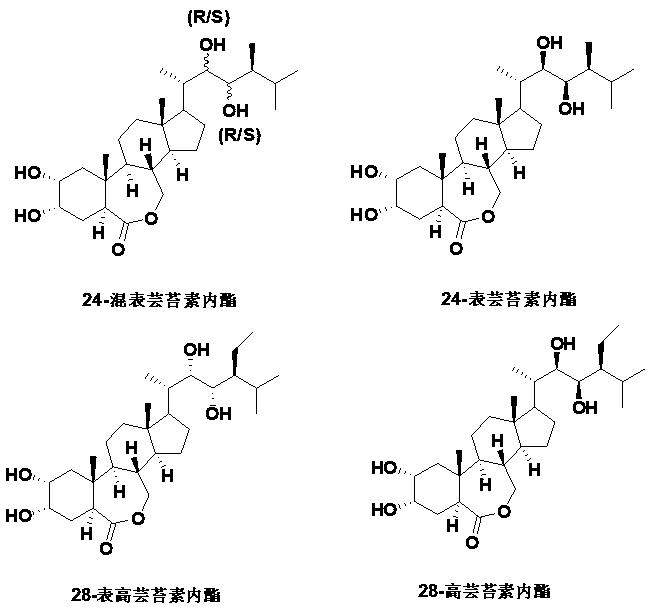
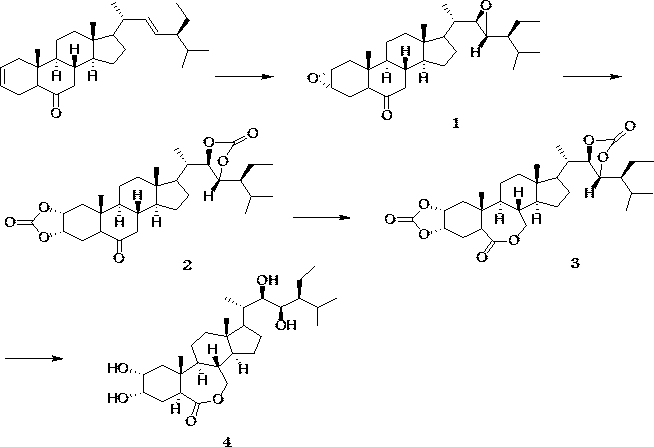
Preparation method with four-step synthesis of 28-homobrassinolide
A technology of steroid lactone and four-step method, which is applied in the field of preparation of four-step synthesis of 28-homobrasinolide, can solve the problems of high preparation cost, large environmental pollution, and difficult industrial production, and achieves a simple and environmentally friendly method , the effect of a wide range of sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (2, 22)-diene-24S-ethyl-5α-cholestan-6-one (410.67 g, 1.00 mol), potassium persulfate (152.00 g, 1.00 mol), fructose ketone (254.32 g , 1.00 mol), water (2 L) were placed in a three-neck flask, and stirred at 25 °C for 5 h. Add ethyl acetate, wash with water, dry, and recover ethyl acetate to obtain product 1: (2 α, 3 α,22 R, 23 R) - 2, 3, 22, 23 - Diepoxy- 24 S - ethyl - 5α-cholestan-6-one. Transfer (2α, 3 α, 22 R, 23 R) - 2, 3, 22, 23 - Diepoxy- 24 S - Ethyl - 5 α - Cholesta-6 -one to a three-neck flask, and add four Butylammonium bromide (32.24 g, 0.10 mol) and water (2 L), at 25 ℃, feed carbon dioxide until the reaction is complete. Add ethyl acetate, wash with water, dry, recover ethyl acetate to obtain product 2: (2 α, 3 α,22 R, 23 R) - 2, 3, 22, 23 - dicarbonate - 24 S - ethyl - 5α-cholestan-6-one. Transfer (2 α, 3 α, 22 R, 23 R) - 2, 3, 22, 23 - dicarbonate - 24 S - ethyl - 5 α - cholestan-6-one to a three-necked flask, and add m-Chloroperbenzoic acid (172....
Embodiment 2
[0029] (2, 22)-diene-24S-ethyl-5α-cholestan-6-one (410.67 g, 1.00 mol), 50% hydrogen peroxide (68.00 g, 1.00 mol), fructose ketone (254.32 g , 1.00 mol), water (2 L) were placed in a three-neck flask, and stirred at 25 °C for 5 h. Add ethyl acetate, wash with water, dry, and recover ethyl acetate to obtain product 1: (2 α, 3α, 22 R, 23 R) - 2, 3, 22, 23 - Diepoxy- 24 S - ethyl- 5 alpha-cholestan-6-one. Transfer (2 α, 3 α, 22 R, 23 R) - 2, 3, 22, 23 - Diepoxy- 24 S - Ethyl - 5 α - Cholester-6-one to a three-neck flask, and add Tetrabutylammonium bromide (32.24 g, 0.10 mol) and water (2 L), at 25 ℃, feed carbon dioxide until the reaction is complete. Add ethyl acetate, wash with water, dry, recover ethyl acetate to obtain product 2: (2 α, 3 α,22 R, 23 R) - 2, 3, 22, 23 - dicarbonate - 24 S - ethyl - 5α-cholestan-6-one. Transfer (2 α, 3 α, 22 R, 23 R) - 2, 3, 22, 23 - dicarbonate - 24 S - ethyl - 5 α - cholestan-6-one to a three-necked flask, and add m-Chloroperbenzoic acid ...
Embodiment 3
[0031] (2, 22)-diene-24S-ethyl-5α-cholestan-6-one (410.67 g, 1.00 mol), potassium persulfate (152.00 g, 1.00 mol), quinidinone ( 324.42 g, 1.00 mol), water (2 L) were placed in a three-necked flask, and stirred at 25 °C for 5 h. Add ethyl acetate, wash with water, dry, and recover ethyl acetate to obtain product 1: (2 α, 3α, 22 R, 23 R) - 2, 3, 22, 23 - Diepoxy- 24 S - ethyl- 5 alpha-cholestan-6-one. Transfer (2 α, 3 α, 22 R, 23 R) - 2, 3, 22, 23 - Diepoxy- 24 S - Ethyl - 5 α - Cholester-6-one to a three-neck flask, and add Tetrabutylammonium bromide (32.24 g, 0.10 mol) and water (2 L), at 25 ℃, feed carbon dioxide until the reaction is complete. Add ethyl acetate, wash with water, dry, recover ethyl acetate to obtain product 2: (2 α, 3 α,22 R, 23 R) - 2, 3, 22, 23 - dicarbonate - 24 S - ethyl - 5α-cholestan-6-one. Transfer (2 α, 3 α, 22 R, 23 R) - 2, 3, 22, 23 - dicarbonate - 24 S - ethyl - 5 α - cholestan-6-one to a three-necked flask, and add m-Chloroperbenzoic acid (1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com