Method for synthesizing ciprofibrate intermediate and the intermediate
A synthesis method and intermediate technology, applied in the synthesis method of ciprofibrate intermediate and the field of intermediates, can solve the problems of easy polymerization, difficulty in reagent transportation and storage, and easy decomposition, so as to improve yield and purity, Avoid the effect of high content of ortho by-products and H2O2
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
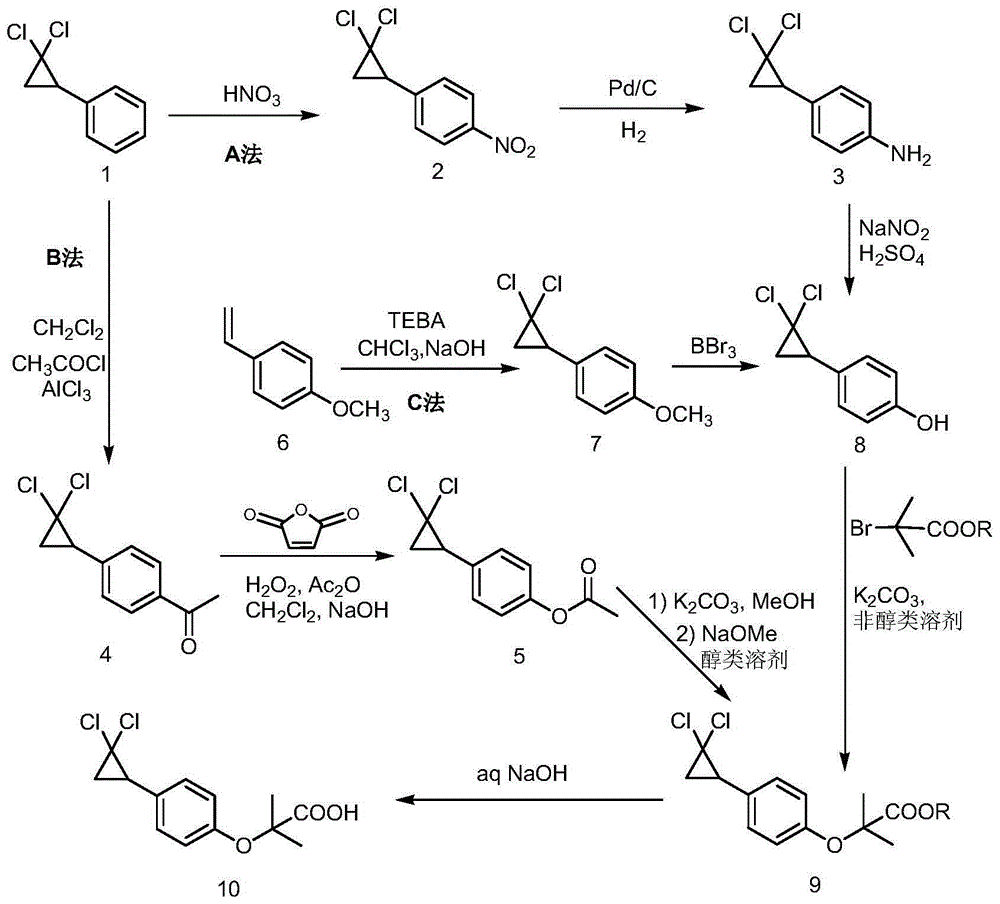
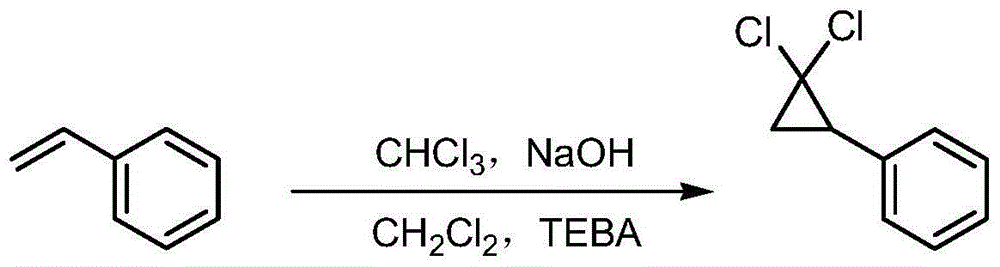
[0043] Embodiment 1: the preparation of 2,2-dichlorocyclopropylbenzene
[0044]
[0045] Add 30g of styrene, 30ml of dichloromethane, 43.5g of chloroform and 1.2g of TEBA (benzyltriethylammonium chloride) into a 250ml four-necked flask. Raise the temperature to reflux, add dropwise lye (28.5g NaOH + 28.5ml water), and react under reflux for 8 hours. Cool to room temperature, add 60ml of water, stir for 10 minutes, let stand to separate layers, extract the water layer with petroleum ether, combine the organic layers, dry over anhydrous sodium sulfate, filter, and distill the filtrate under high vacuum with an oil pump, slowly heat up to distillate After stabilization, control the temperature at about 95-100°C for distillation to obtain a colorless liquid. Yield: 88.6%; HPLC purity: 99.2%. The HPLC peak time of the product is consistent with that of a commercially available standard sample (purchased from Energy Chemical).
Embodiment 2
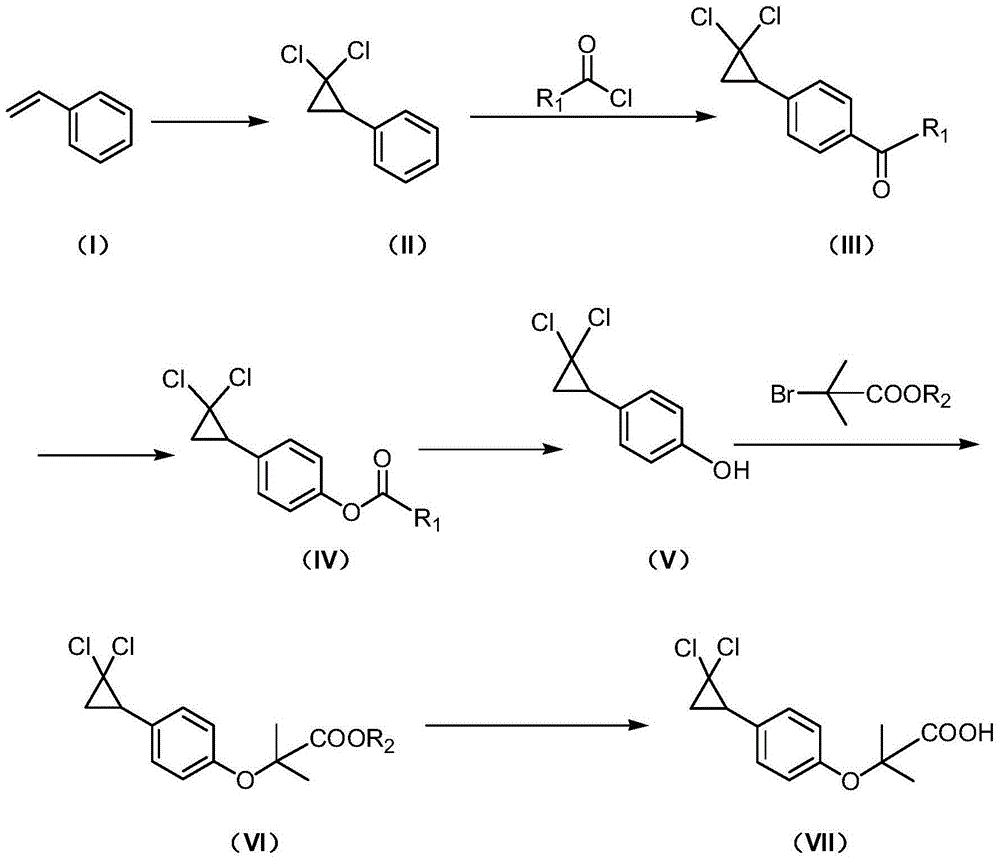
[0046] Example 2: Preparation of 1-[4-(2,2-dichlorocyclopropyl)phenyl]-1-hexanone
[0047]
[0048] Add 210ml of dichloromethane, 60g of AlCl to a dry 500ml four-neck flask 3 , control the temperature at 20°C, slowly add 75.4g of n-hexanoyl chloride dropwise under stirring, control the temperature below 30°C, continue stirring until AlCl 3 completely dissolved. Cool to 20-25°C, slowly add 70g of 2,2-dichlorocyclopropylbenzene dropwise, raise the temperature to 30-35°C after the dropwise addition, and keep the reaction for 3 hours. After the reaction, the reaction solution was added dropwise to 370 g of ice water, the organic phase was separated, washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain 1-[4-(2,2-dichlorocyclo Propyl)phenyl]-1-hexanone. Yield: 98%.
[0049] end product from 1 H-NMR, mass spectrometry and HPLC characterization.
[0050] HPLC purity: 98.3%, wherein the...
Embodiment 3
[0055] Embodiment 3: Preparation of 4-(2,2-dichlorocyclopropyl)phenol
[0056]
[0057] Add 520ml of acetic acid into a 1000ml four-neck flask, cool to 10-15°C, add 138g of carbamide peroxide (UHP), add 118g of maleic anhydride, and keep stirring at 15-20°C for 3 hours. Raise the temperature to 35-40°C, add 86.1g of 1-[4-(2,2-dichlorocyclopropyl)phenyl]-1-hexanone dropwise, raise the temperature to 45-50°C after dropping, and keep the reaction for 5 hours. Cool to room temperature, wash with water, dry over anhydrous sodium sulfate, filter, and concentrate under reduced pressure to obtain a brown-yellow liquid.
[0058] Add 100ml of methanol and 2.6g of potassium carbonate, heat up to 35-40°C with stirring, keep the reaction for 4 hours, and concentrate under reduced pressure to remove methanol. The concentrate was dissolved in dichloromethane, washed with water, adjusted to pH 9-10 with dilute lye, the aqueous layer was separated, acidified with dilute hydrochloric acid t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com