ε-n-pentyl propionate-ε-caprolactone and its preparation method
A technology of n-pentyl propionate and n-pentyl acrylate, applied to the field of lactone-type fragrance compounds, ε-n-pentyl propionate-ε-caprolactone and its preparation, can solve the problem of low yield and low cost High, difficult ε-lactone type fragrance compounds and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A kind of synthetic method of n-pentyl acrylate compound, concrete steps are as follows:
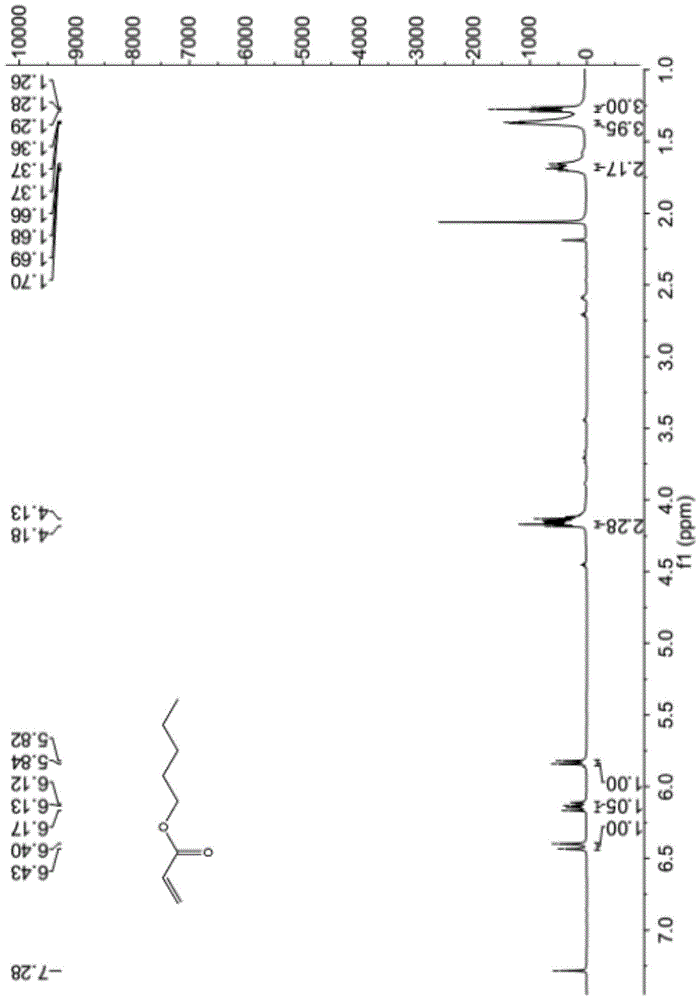
[0033] In a 100ml round bottom flask, add 17.32g (0.19mol) of anhydrous toluene, 4.9g (0.01mol) of p-toluenesulfonic acid, 7.245g (0.10mol) of acrylic acid, 11.02g (0.13mol) of n-pentanol in Magnetic stirring and reflux at 110°C for 8 hours of reaction; after the reaction was completed, the reaction solution was cooled to room temperature, saturated aqueous sodium bicarbonate solution was added to the reaction solution, the organic phase was extracted with ethyl acetate, and the organic phase was washed three times with saturated brine. Anhydrous magnesium sulfate was dried, and the solvent was removed by rotary evaporation with a rotary evaporator. The product was obtained by thin-layer chromatography, and the eluent was PE:EA=8:1. The pure product was collected to finally obtain 12.87ml of transparent and colorless liquid. The rate is 94.2%.
[0034] The transparent colorless l...
Embodiment 2
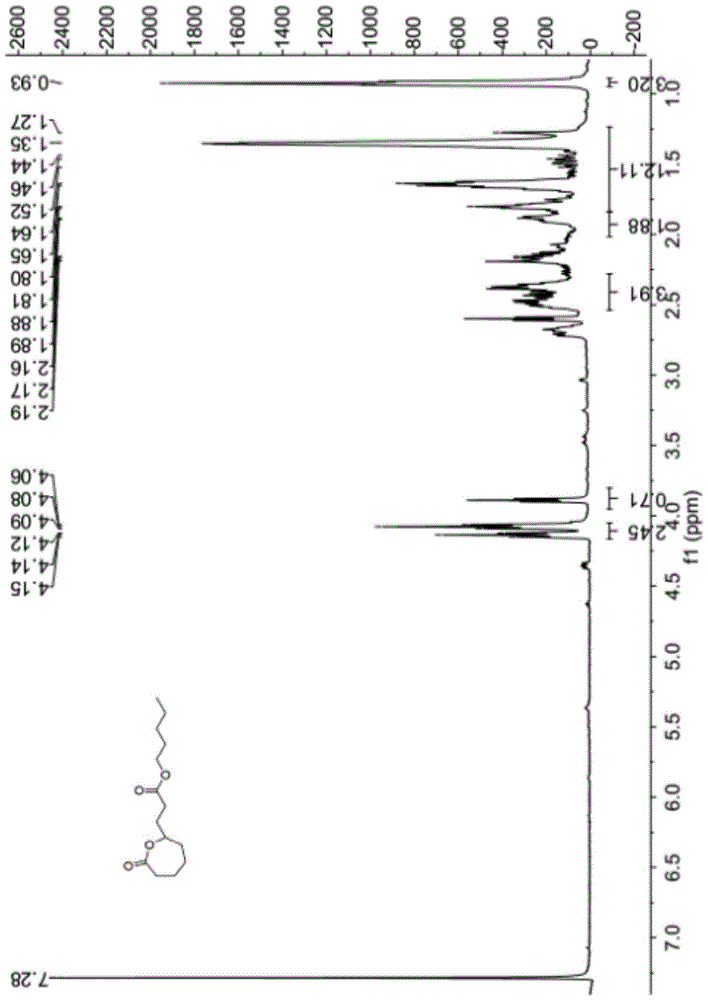
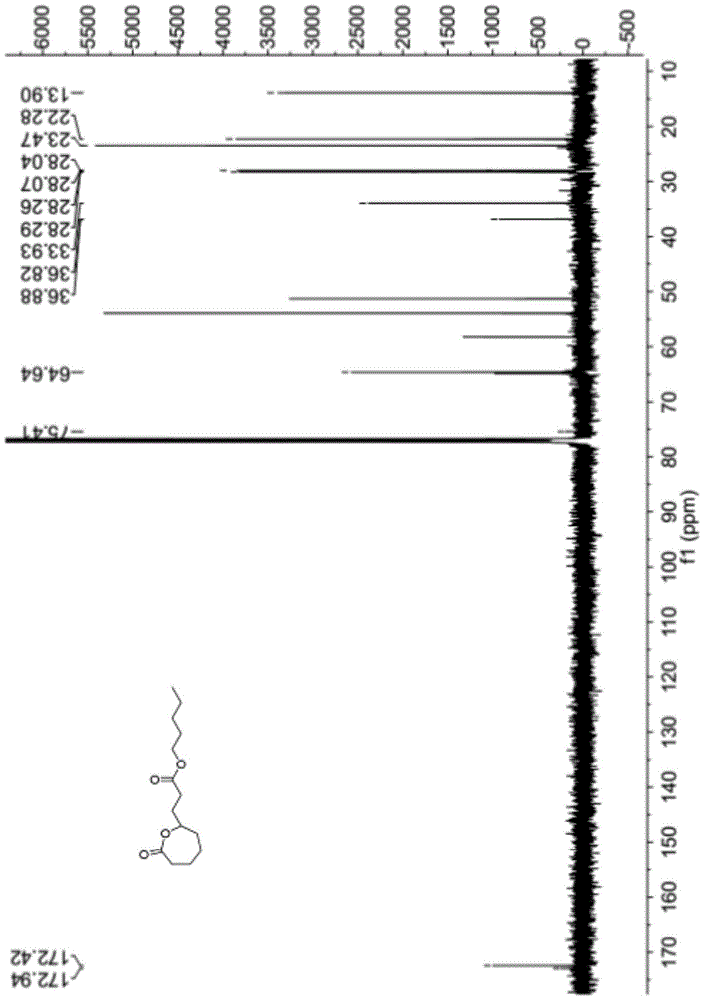
[0038] The synthetic method of above-mentioned a kind of ε-propionate n-pentyl-ε-caprolactone compound, concrete steps are as follows:
[0039] Add 1.42g (0.01mol) of disodium hydrogen phosphate solid into a 50ml three-necked flask, replace with nitrogen three times, inject 13.25g (0.16mol) of dichloromethane, and inject 1.85g (0.013mol) of n-pentyl acrylate, 0.97 g (0.0064mol) 1-pyrrolidine-1-cyclohexene, after stirring for 30 minutes, slowly inject 0.86g (0.005mol) m-chloroperoxybenzoic acid solution dissolved in 13.25g (0.16mol) methylene chloride, in Complete within 10 minutes, under magnetic stirring, react for 4 hours; after the reaction is completed, put the three-neck flask into ice water to cool, add 15ml of normal temperature saturated sodium bicarbonate aqueous solution to the reaction solution to wash once, and wash twice with 30ml saturated sodium thiosulfate aqueous solution , washed twice with 95ml of 5% sodium hydroxide aqueous solution, twice with 50ml of dist...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com