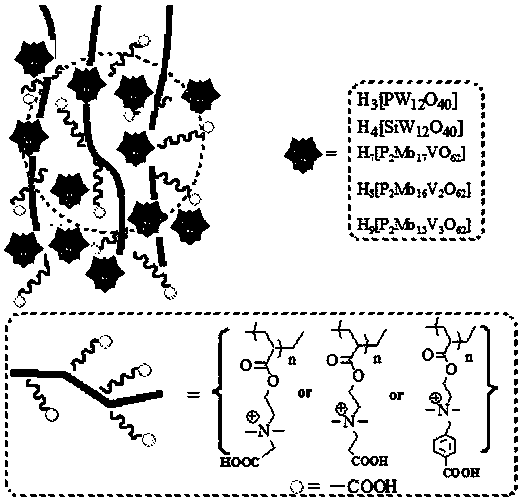
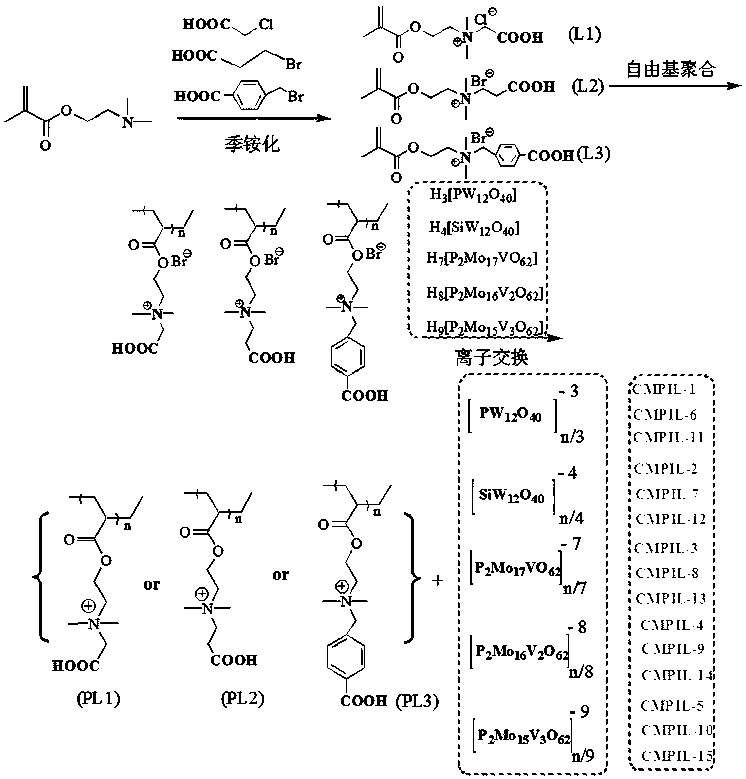
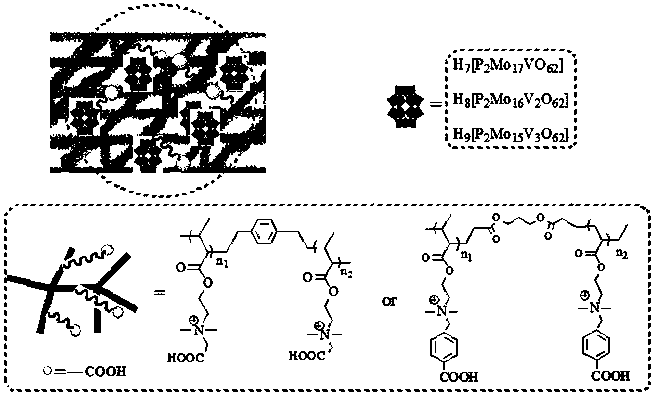
Carboxyl functionalized porous heteropoly acid polyionic liquid and use thereof
A polyionic liquid, carboxyl functionalization technology, applied in organic chemistry, chemical instruments and methods, chemical/physical processes, etc., can solve problems such as thermal instability of catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: N, N- Dimethyl- N -Carboxymethyl- N- Synthesis of Ethyl Methacrylate Quaternary Ammonium Salt (L1)
[0051] Under the protection of nitrogen, add 6mL dimethylaminoethyl methacrylate and 25 mL acetone in turn to the reaction flask, stir well and then raise the temperature to 50°C, add 3.5g chloroacetic acid in three times, and keep it warm for 24h after the addition; After cooling to room temperature, the solvent acetone was removed by rotary evaporation, and then vacuum-dried to constant weight to obtain N, N- Dimethyl- N -Carboxymethyl- N- Ethyl methacrylate quaternary ammonium salt, its productive rate is 97.3%.
[0052] FT-IR(KBr), ν / cm -1 : 3567, 2972, 1716, 1622, 1381.
Embodiment 2
[0053] Example 2: N, N- Dimethyl- N -Carboxyethyl- N- Synthesis of Ethyl Methacrylate Quaternary Ammonium Salt (L2)
[0054] 3.5g chloroacetic acid was replaced by 4.0g 3-bromopropionic acid, others were the same as in Example 1, and the target product yield was 95.4%.
[0055] FT-IR(KBr), ν / cm -1 : 3420, 2960, 1728, 1631, 1390.
Embodiment 3
[0056] Example 3: N, N- Dimethyl- N -(4-carboxy)benzyl- N- Synthesis of Ethyl Methacrylate Quaternary Ammonium Salt (L3)
[0057] 3.5g chloroacetic acid was replaced by 6.1g 4-bromomethylbenzoic acid, and others were the same as in Example 1, and the target product yield was 96.5%.
[0058] FT-IR(KBr), ν / cm -1 : 3475, 3014, 2972, 1716, 1622, 1595, 1498, 1381.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com