Baeyer-Villiger oxidation reaction catalyst and preparation method and application thereof
A technology of oxidation reaction and catalyst, applied in the direction of physical/chemical process catalyst, catalytic reaction, organic compound/hydride/coordination complex catalyst, etc. Explosion and other problems, to achieve the effect of novel catalyst structure, constant catalytic activity and easy recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
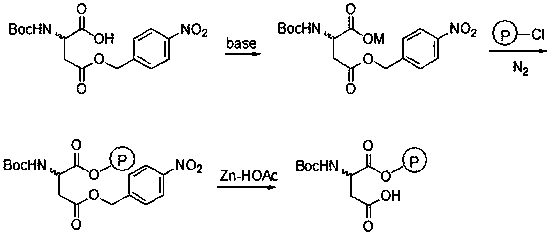
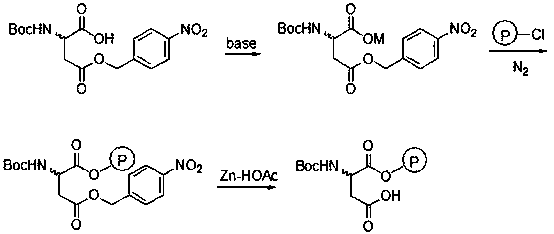
[0021] In a 250mL four-neck flask, add 50mL of water, 2.33g (0.012mol) of CsHCO 3 and 50mL of methanol, stirred, added 3.09g (0.01mol) N-Boc-aspartic acid-4-(4-nitro)benzyl ester, reacted at room temperature for 10min, and added 0.65g (0.003mol) CsHCO 3 , control the pH of the system to 8-9, after the reaction is completed, the methanol is distilled off and dried to obtain 4.40 g of white cesium salt of N-Boc-aspartic acid-4-(4-nitro)benzyl ester.
[0022] In 20 mL of a mixed solvent of DMF and N-methylpyrrolidone (V / V=13:7), add 0.62 g (1.4 mmol) of cesium salt of N-Boc-aspartic acid-4-(4-nitro)benzyl ester , 1.0g Merrifield resin (chlorine content: 0.7mmol / g), N 2 Under protection, stir the reaction at 50°C for 36h, filter, and wash the resin with 5mL×3H 2 O, washed with 5 mL×3 DMF, 5 mL×3 dichloromethane, and dried under vacuum to obtain 1.18 g of the product.
[0023] Add 1.0 g of the resin prepared above into 10 mL of glacial acetic acid (mass fraction: 90%), cool to 0...
Embodiment 2
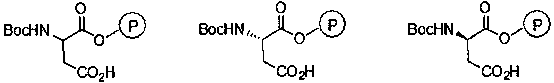
[0026] Substitute (S)-N-tert-butoxycarbonyl-aspartic acid-4-(4-nitro)benzyl ester for N-tert-butoxycarbonyl-aspartic acid-4-(4-nitro)benzyl ester , and other operations were the same as in Example 1 to obtain (S)-N-tert-butoxycarbonyl-aspartic acid catalyst supported by Merrifield resin, with a loading capacity of 0.66 mmol / g.
Embodiment 3
[0028] Substitute (R)-N-tert-butoxycarbonyl-aspartic acid-4-(4-nitro)benzyl ester for N-tert-butoxycarbonyl-aspartic acid-4-(4-nitro)benzyl ester , and other operations were the same as in Example 1 to obtain (R)-N-tert-butoxycarbonyl-aspartic acid catalyst supported by Merrifield resin, with a loading capacity of 0.67 mmol / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com