1,2-glycoside transderivative of oxazole compounds and preparation method thereof
A technology for glycoside derivatives and compounds, which is applied in the first field of oxazole compounds, can solve the problems of high lipophilicity, poor water solubility, low bioavailability and the like, and achieves a short route, good water solubility and simple preparation method. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
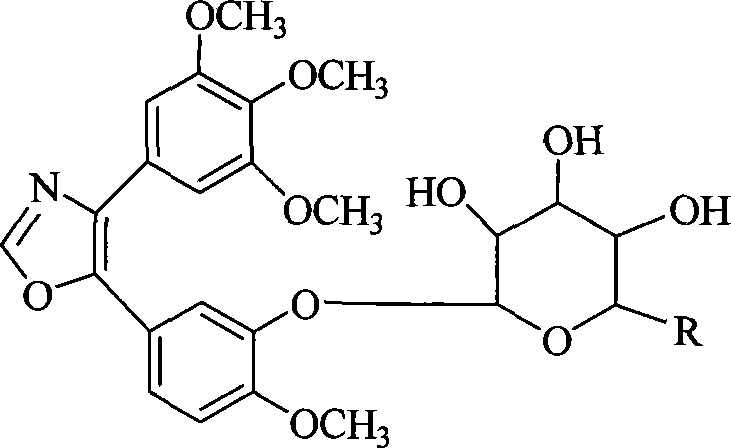
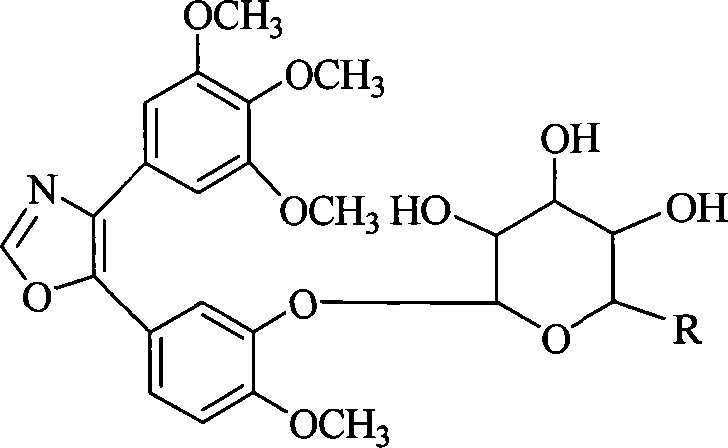
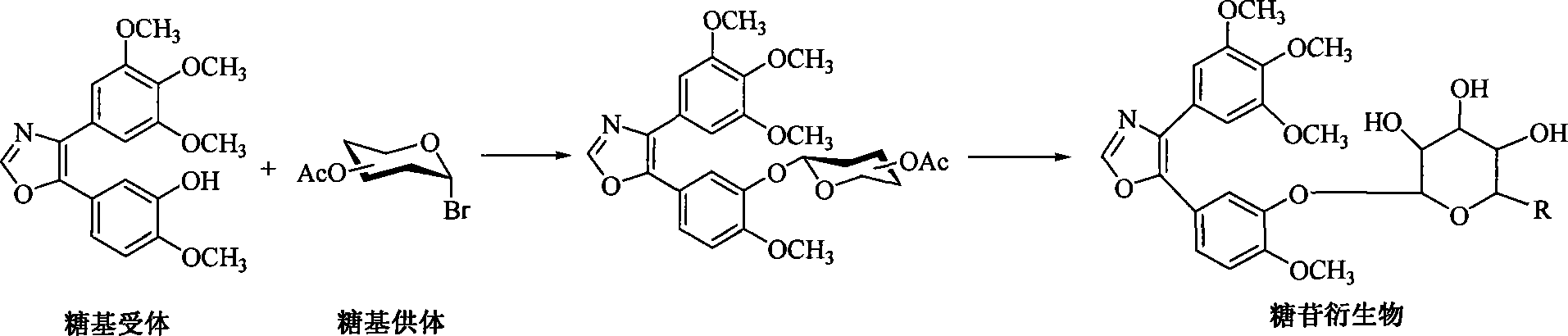
[0010] Oxazole analogue 4-(3,4,5-trimethoxyphenyl)-5-(3-hydroxy-4-methoxyphenyl) of Lamellarin 1C and CA-4 with simple structure and significant antitumor activity ) Oxazole has a certain skeleton similarity in structure, and the oxazole analog of CA-4 is equivalent to replacing the pyrrole ring at the center of the Lamellarin skeleton with an oxazole ring. Therefore, the present invention uses the oxazole analogs of CA-4 to simulate Lamellarins as a lead compound, conducts structural modification, and prepares a highly active compound that inhibits microtubule aggregation and selectively targets endothelial cells. A microtubule-binding anti-tumor drug with a novel structure and obvious destructive effect on tumor blood vessels.
[0011] The present invention uses glycosylation to modify the lead compound, which has good significance for improving its water solubility, bioavailability and improving the targeting of drug molecules.
[0012] 1. The preparation of D-glucose, D-g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com