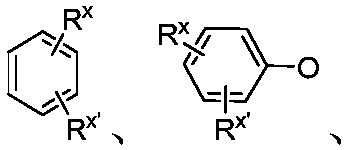
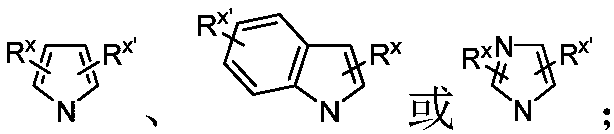
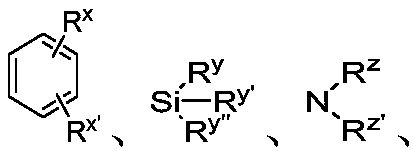Chiral single phosphorus ligand PC-Phos based on xanthene framework, preparation method of full structure of ligand and application
A technology of xanthene and pc-phos is applied in the field of novel chiral monophosphine ligands and their preparation, which can solve the problems of high toxicity of reagents, lengthy synthesis routes, expensive raw materials and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11a
[0120] Example 1 1a(S,R s ) synthesis (reference scheme 1)
[0121] Step 1: In a 250mL dry single-necked bottle, add under nitrogen atmosphere (20mmol,
[0122] 9.24g) and 60mL THF, stirred at -78°C for 10 minutes, added dropwise n-BuLi (1.0eq., 12.5mL, 1.6M), continued to stir for 1.5 hours, added dropwise ClPPh 2 (1.0eq., 3.57mL), stirred for another 1 hour, added dropwise n-BuLi (2.0eq., 25mL, 1.6M), stirred for another 1.5 hours, added dropwise DMF (15eq., 20mL), and slowly rose to room temperature Continue to stir for 1 hour, separate the layers, extract the aqueous layer three times with ethyl acetate, combine the organic phases, wash with water and saturated sodium chloride respectively, dry over anhydrous sodium sulfate, filter, spin dry, and purify by column chromatography to obtain The yield was 65%. 1 H NMR (400MHz, CDCl 3 ):δ10.17(s,1H),7.68-7.60(m,2H),7.45(d,J=7.6Hz,1H),7.38-7.30(m,10H),7.11(t,J=9.0Hz, 1H), 7.05(t, J=7.6Hz, 1H), 1.65(s, 6H); 31 P NMR (1...
Embodiment 2
[0127] Example 2 The synthesis (reference scheme one)
[0128] Concrete operation with reference to embodiment 1, raw material used is The yield was 99%. 1 H NMR (500MHz, CDCl 3 )δ7.50-7.26(m,13H),7.21(d,J=6.5Hz,1H),7.12(t,J=8.0Hz,1H),7.02(t,J=7.5Hz,1H),6.70- 6.55(m,3H),6.05(br,1H),1.69(s,3H),1.63(s,3H),1.29(s,9H); 13 C NMR (125MHz, CDCl 3 )δ158.50, 152.38 (J c,p =17Hz), 147.70, 137.09 (J c,p =11Hz), 136.35, 134.03, 133.95, 133.74 (J c,p =19Hz), 133.32, 132.22, 130.57, 130.00 (J c,p =2Hz), 129.06, 128.60, 128.53, 126.97, 125.19, 123.55, 123.24, 113.44, 56.21, 34.43, 32.35, 22.99; 31 P NMR (202MHz, CDCl 3 )δ-17.81; HRMS (ESI) calculated for [C 32 h 33 NO 2 PS][M+H] + :526.1964; found: 526.1959.
Embodiment 31b
[0129] Example 3 1b(R,R s ) synthesis (reference scheme 1)
[0130] Referring to Example 1 for the specific operation, the metal reagent used is 4-methoxyphenyllithium, and the yield is 83%. 1 H NMR (500MHz, CDCl 3 )δ7.49-7.34(m,10H),7.34-7.25(m,4H),7.22-7.11(m,4H),7.11-6.99(m,2H),6.65(s,1H),6.23(br, 1H), 1.69(s,3H), 1.61(s,3H), 1.28(s,9H), 1.21(s,9H).; 13 CNMR (125MHz, CDCl 3 )δ152.51(J c,p =17Hz), 152.45, 149.64, 148.00, 139.06, 137.0 (J c,p =12Hz), 136.7(J c,p =11Hz), 134.3(J c,p =21Hz), 133.6(J c,p =20Hz), 132.15, 130.57, 130.1 (J c,p =10Hz), 128.76, 128.51 (J c,p =13Hz), 128.51, 128.41, 127.22, 126.71, 125.15, 124.74, 123.2 (J c,p =65Hz), 56.12, 34.50, 34.37, 32.48, 31.36, 22.81; 31 P NMR (202MHz, CDCl 3 )δ-17.95; HRMS (ESI) calculated for [C 42 h 47 NO 2 PS][M+H] + :660.3060,found:660.3054.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com