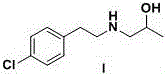
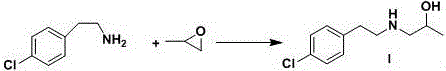
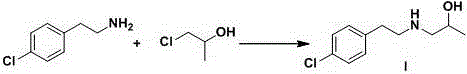
Preparation method of lorcaserin intermediate
A technology for lorcaserin and intermediates, which is applied in the field of preparation of lorcaserin intermediates, can solve the problems of high price, high toxicity of HBr, complicated operation and the like, and achieves easy storage and use, avoidance of industrial waste water, and reaction operation. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the synthesis of p-chlorophenethylamine
[0041]
[0042] Add 50.0g of p-chlorophenylacetonitrile, 22.0g of sodium borohydride and 500ml of tetrahydrofuran into a 1000ml four-neck flask, stir under nitrogen protection, and cool down in an ice-salt bath. When the system temperature is 0~5°C, add 88.4g of boron trifluoride ether Add it into a 1000mL four-necked bottle through a constant pressure dropping funnel. During the dropping process, control the temperature of the reaction system at 0-10°C. A large amount of gas will be generated during the dropping process. After the dropping is completed, a large amount of white solid will be generated in the reaction system. Heat up to 25~35°C and stir for 1 hour, then raise the temperature to 45~55°C and stir for 4~6 hours, follow the reaction by HPLC until the content of p-chlorophenylacetonitrile in the raw material is less than 1.0%, stop heating, cool down to 0~5°C, pass constant pressure Add 20ml of water ...
Embodiment 2
[0043] Embodiment 2: the synthesis of p-chlorophenethylamine
[0044]
[0045]Add 50.0g of p-chlorophenylacetonitrile, 20.0g of sodium borohydride and 500ml of 2-methyltetrahydrofuran into a 1000ml four-necked flask, stir under nitrogen protection, and cool down in an ice-salt bath. When the system temperature is 0~5°C, add 75.0g of Boron fluoride ether was added into a 1000ml four-necked bottle through a constant pressure dropping funnel. During the dropping process, the temperature of the reaction system was controlled at 0-10°C. A large amount of gas was generated during the dropping process. After the dropping, the reaction system had a large amount of gas. A white solid is formed, heat up to 25~35°C and stir for 1 hour, then heat up to 45~55°C and stir for 4~6 hours, follow the reaction by HPLC until the content of p-chlorophenylacetonitrile in the raw material is less than 1.0%, stop heating, and cool down to 0~5°C , add 20ml of water dropwise through the constant pre...
Embodiment 3
[0046] Embodiment 3: Synthesis of lorcaserin intermediate I
[0047]
[0048] Add 30.0g of 1-chloro-2-propanol, 88.0g of potassium carbonate and 500ml of acetonitrile into a 1000ml four-neck flask, stir under nitrogen protection, control the temperature of the system at 20~25°C, and constant Add the pressure drop funnel into a 1000ml four-necked bottle, control the dropping speed, and control it at 30.0~40.0min. After the dropwise addition is completed, raise the temperature to 55~60°C and stir for 8~10 hours. HPLC will track the reaction until the content of p-chlorophenethylamine in the raw material is reached. Less than 1.0%, remove acetonitrile by distillation under reduced pressure, add 300ml water and 500ml ethyl acetate to extract, separate layers, remove ethyl acetate by distillation under reduced pressure to obtain a light yellow solid, and the solid is recrystallized with ethyl acetate and n-heptane (ethyl acetate The volume ratio of n-heptane and n-heptane is 1:8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com