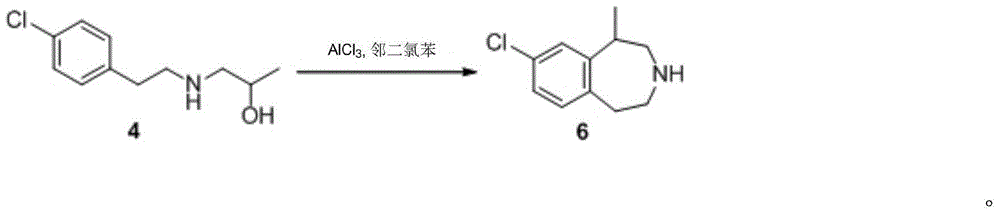
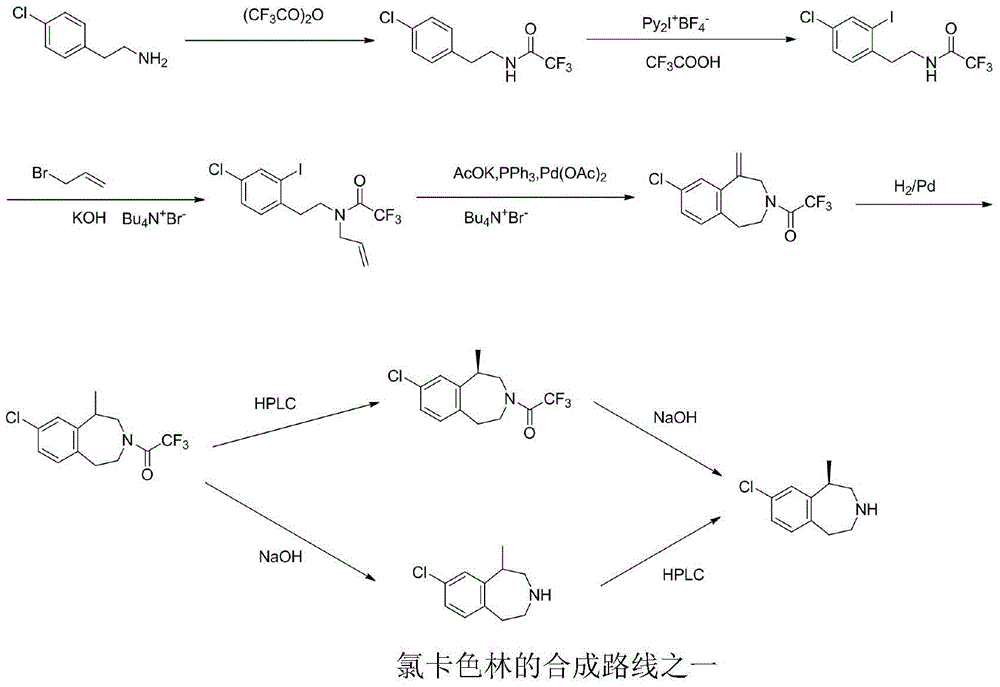
A preparing method of (R,S)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine, a lorcaserin intermediate
A technology of lorcaserin and intermediates, which is applied in the field of preparation of slimming drug lorcaserin, can solve the problems of equipment causing corrosion, long Friedel-Crafts reaction time, and waste acid polluting the environment, etc., so as to save energy and reduce steps, environment friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
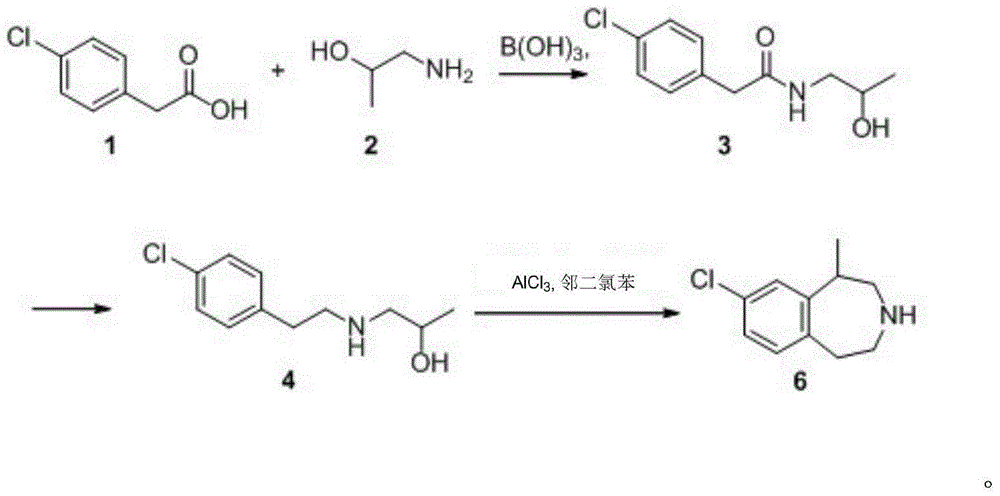
[0049] One of the preparation methods of 2-(4-chlorophenyl)N-(2-hydroxypropyl)acetamide (compound 3)
[0050]Compound 1 (100.0g, 0.59mol), boric acid (3.7g, 0.06mol, 0.1eq) and 300ml of toluene were sequentially added to a 1L three-necked flask, heated to 40°C, and then oil compound 2 (47.87g, 0.64mol, 1.1eq) and 200ml of toluene mixed solution, heated to reflux at 135°C, after 24h, the amount of water in the water separator no longer increased, and the reaction temperature dropped to 60°C. The reaction solution was slowly poured into an aqueous solution of sodium carbonate with a weight concentration of 5%, stirred, and a large amount of white solids were precipitated, cooled to room temperature, filtered, and dried in a filter cake blast drying oven for 3 hours to obtain 122.8 g of white granular solids, yield 92.0%, HPLC purity 95.5%. 1 H-NMR (400MHz, DMSO-d6) δ: 8.06 (s, 1H); 7.38 (d, 2H, J = 8.7Hz); 7.30 (m, 2H); 7.82 (d, 2H, J = 8.7Hz); 4.71(d,1H,J=1.8Hz),3.64(m,1H),3....
Embodiment 2
[0051] Example 2 The second preparation method of 2-(4-chlorophenyl)N-(2-hydroxypropyl)acetamide (compound 3)
[0052] Compound 1 (60.0g, 0.35mol), boric acid (1.1g, 0.06mol, 0.05eq) and 180ml of toluene were sequentially added to a 500mL three-necked flask, heated to 40°C, and then oil compound 2 (29.12g, 0.39mol, 1.1eq) and 120ml of toluene, heated to reflux at 135°C, after 24h, the amount of water in the water separator no longer increased, and the reaction temperature dropped to 60°C. The reaction solution was slowly poured into 5% sodium carbonate aqueous solution, stirred, and a large amount of white solids were precipitated, cooled to room temperature, filtered, and dried in a filter cake drying oven for 3 hours to obtain 75.5 g of white granular solids, with a yield of 95.0% , HPLC purity 96.5%.
Embodiment 3
[0054] The third preparation method of 2-(4-chlorophenyl)N-(2-hydroxypropyl)acetamide (compound 3)
[0055] Compound 1 (100.0g, 0.59mol), boric acid (3.7g, 0.06mol, 0.1eq) and 300ml of toluene were sequentially added to a 1L three-necked flask, heated to 40°C, and then oil compound 2 (47.87g, A mixed solution of 0.64mol, 1.1eq) and 200ml toluene was heated to reflux at 135°C. After 48h, the amount of water in the water separator no longer increased, and the reaction temperature dropped to 60°C. The reaction solution was slowly poured into 5% sodium carbonate aqueous solution, stirred, and a large amount of white solids were precipitated, cooled to room temperature, filtered, and dried in a filter cake blast drying oven for 3 hours to obtain 123.6 g of white granular solids, with a yield of 92.3% , HPLC purity 96.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com