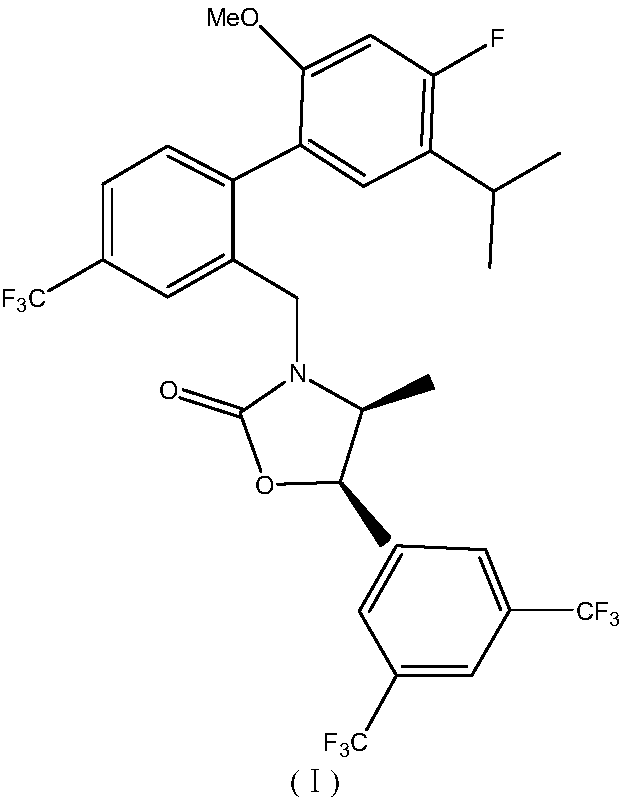
Patents
Literature
46 results about "Borane dimethylsulfide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Borane dimethylsulfide (BMS) is a complexed borane reagent that is used for hydroborations and reductions. The advantages of BMS over other borane reagents, such as borane-tetrahydrofuran, are its increased stability and higher solubility. BMS is commercially available at much higher concentrations than its tetrahydrofuran counterpart (10 M neat) and does not require sodium borohydride as a stabilizer, which could result in undesired side reactions. In contrast, borane·THF requires sodium borohydride to inhibit reduction of THF to tributyl borate. BMS is soluble in most aprotic solvents.
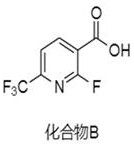
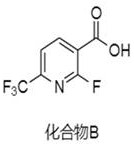
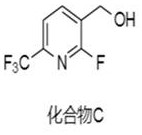
Synthesis method of (2-fluoro-6-(trifluoromethyl) pyridine-3-yl) methanol
Owner:阿里生物新材料(常州)有限公司
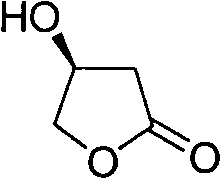
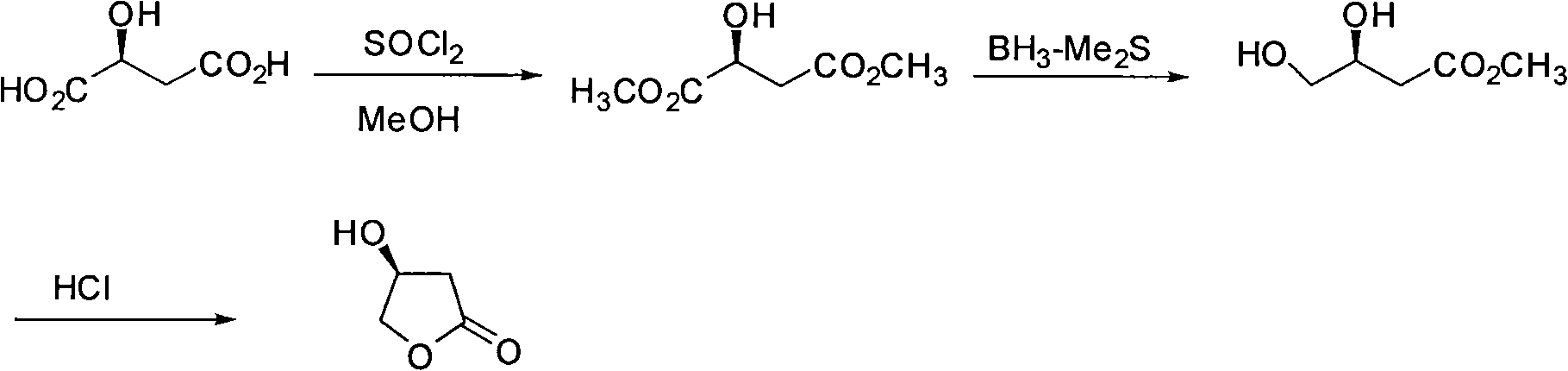
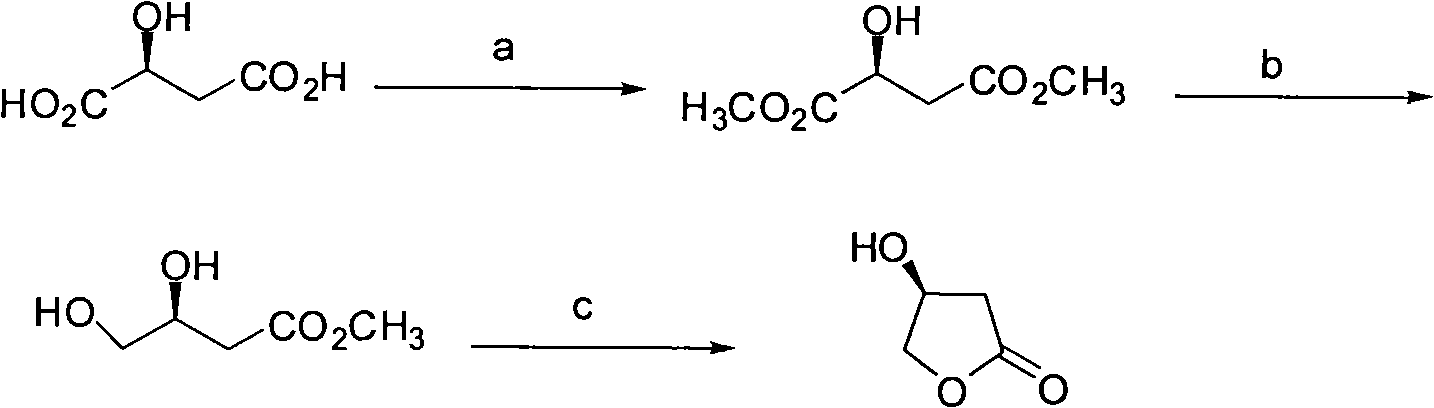
Synthesis method of S-beta-hydroxy-gamma-butyrolactone
The invention discloses a synthesis method of S-beta-hydroxy-gamma-butyrolactone, comprising the steps of: synthesis of L-dimethyl ester malate, synthesis of 3, 4-dihydroxybutyric acid methyl ester, synthesis of S-hydroxy-gamma-butyrolactone and the like.The synthesis method mainly adopts BiBr3 / hydroborate / lower alcohol reducing system to selectively reduce monoester into alcohol, so that borane dimethyl sulfide composite which is combustible and explosive, is not easily stored and has high price in the prior art is avoided being used. The synthesis method of the S-beta-hydroxy-gamma-butyrolactone is suitable for industrialized production.
Owner:SHANGHAI INST OF TECH
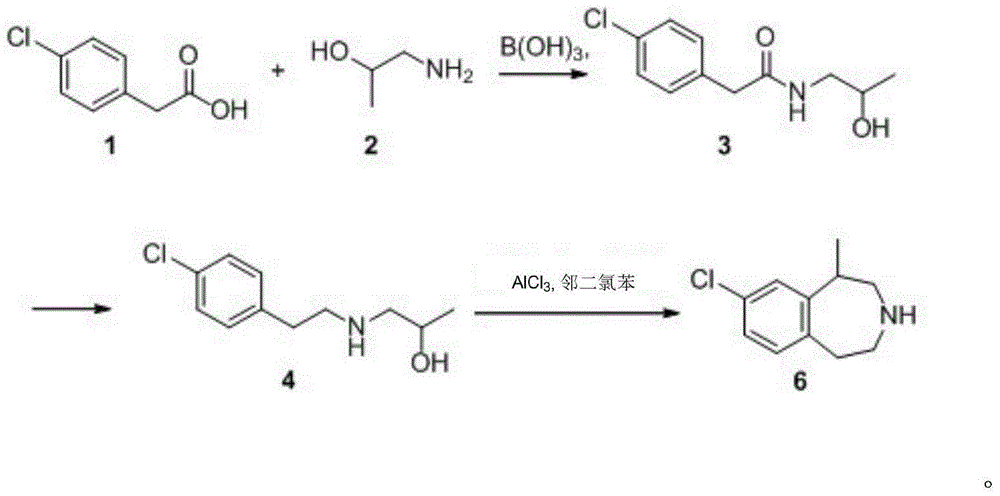
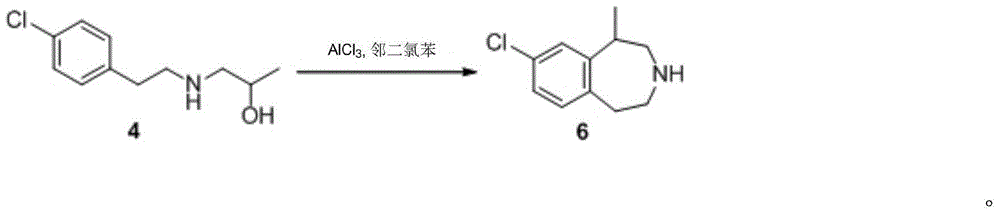
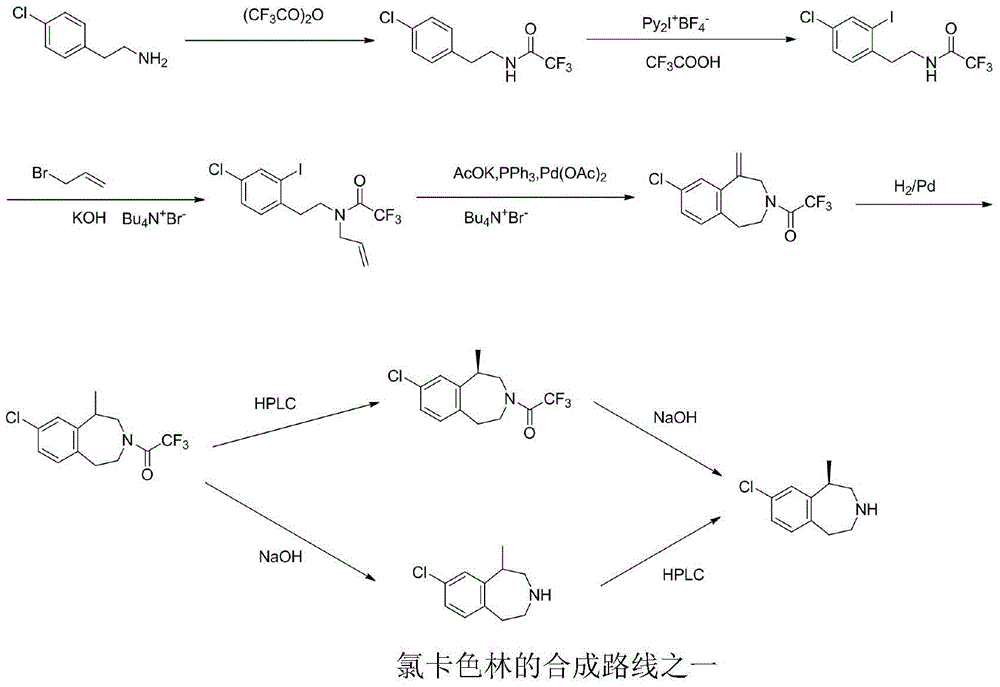
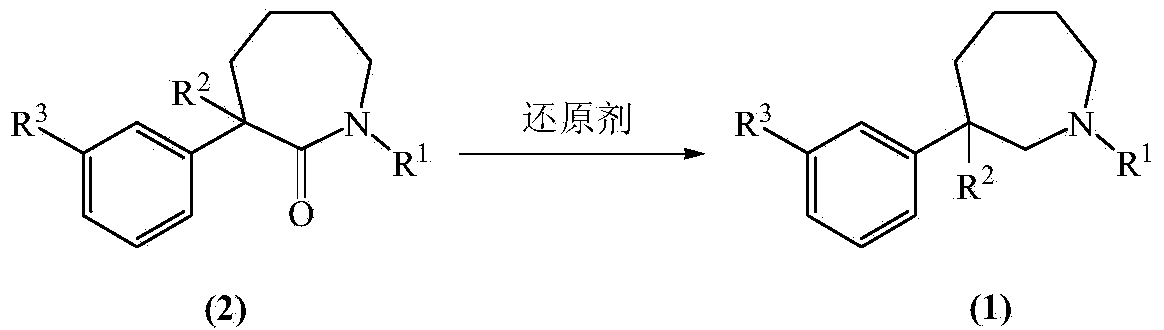
A preparing method of (R,S)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine, a lorcaserin intermediate
ActiveCN105693610AReduce usageLow costOrganic compound preparationAmino-hyroxy compound preparationMethyl groupRaw material
A preparing method of (R,S)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine that is a lorcaserin intermediate is disclosed. The method includes (1) dehydrating a compound 1 and a compound 2 under catalysis by boric acid to generate an amide 3, (2) reducing the intermediate 3 with a borane dimethylsulfide complex to obtain a compound 4, and (3) subjecting the compound 4 to direct ring closing with the existence of aluminum chloride to generate the lorcaserin intermediate that is the (R,S)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine that is the compound 6. The method avoids use of 3,4,5-trimethoxyphenylboronic acid that is an expensive reagent, thus reducing the cost. The method avoids use of thionyl chloride so that the method is environmental friendly. A step of hydroxy chlorination is reduced so that the method is simple in process. The conversion ratio of raw materials and the total yield of reactions are increased. The yield of the compound 6 is increased from 60% in a patent to 82%. The method is suitable for industrial production. A reaction equation is shown in the description.
Owner:SICHUAN YIBIN WULIANGYE GROUP YIBIN PHARMA +1
Preparation method of chiral S/R-3-ethoxy-4-methoxy-alpha[(methylsulfonyl)methyl] benzyl alcohol
ActiveCN105461602AShort reaction timeReduce waste disposalCarboxylic acid nitrile preparationOrganic compound preparationBENZYL ALCOHOL/WATERBromoethane
The invention relates to a preparation method of chiral S / R-3-ethoxy-4-methoxy-alpha[(methylsulfonyl)methyl] benzyl alcohol. The preparation method comprises the following steps: 3-hydroxy-4-methoxybenzaldehyde is taken as a starting material and reacts with hydroxylammonium hydrochloride to produce 3-hydroxy-4-methoxybenzonitrile; 3-hydroxy-4-methoxybenzonitrile reacts with bromoethane to produce 3-ethoxy-4-methoxybenzonitrile; 3-ethoxy-4-methoxybenzonitrile reacts with dimethyl sulfone under the action of n-butyllithium, a product is hydrolyzed in an aqueous hydrochloric acid solution, and 1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethanone is obtained; finally, S-(-)-alpha,alpha-diphenyl-2-pyrrolidinemethanol or R-(+)-alpha,alpha-diphenyl-2-pyrrolidinemethanol is taken as a chiral catalyst, a borane dimethyl sulfide solution is taken as a reducing agent, carbonyl is reduced, and a product is obtained. The reaction conditions are mild, the product yield is higher, the technology level is increased, the operability is improved, and large-scale industrial production is facilitated.
Owner:DONGHUA UNIV
Preparation method of sitafloxacin side chain intermediate
InactiveCN104230790AHigh yieldImprove securityOrganic chemistryBulk chemical productionSide chainEthylamine
The invention relates to a preparation method of a sitafloxacin side chain intermediate. The preparation method comprises the following steps: synthesizing 4-methyl acetoacetate with 1,2-dibromoethane into a three-membered ring, synthesizing the three-membered ring with ammonia water into nitrogen heterocycle, synthesizing nitrogen heterocycle with R-1-phenyl ethylamine to generate chiral carbon, reducing through sodium borohydride, adding ditertbutyl dicarbonate to generate a protecting group, and finally performing carbonyl reduction to borane-dimethyl sulfide to obtain an intermediate. According to the preparation method, the atom utilization rate can be improved, the use of toxic reagents can be reduced, and the amplification of the technology can be facilitated.
Owner:SUZHOU NACHI BIOTECH CO LTD
Liquid hydrocarbon fuel low-temperature self-ignition adjusting method based on atomic carrier
ActiveCN111534338AAchieve low temperature self-ignitionEasy to operateLiquid carbonaceous fuelsFuel additivesSpontaneous combustionKerosene
The invention discloses a liquid hydrocarbon fuel low-temperature self-ignition adjusting method based on an atomic carrier, and relates to the field of aviation fuels. Through the atom carrier additive, the combustion energy barrier of a substrate fuel is reduced, and low-temperature self-ignition of the hydrocarbon fuel is realized; the atomic carrier additive is a borane dimethyl sulfide complex; the substrate fuel is mixed fuel of octene, hexene or olefin and RP-3 kerosene. The method has the advantages of real-time ignition regulation and control, low self-ignition temperature, high safety and the like, the low-temperature spontaneous combustion characteristic of the liquid hydrocarbon fuel can be efficiently regulated and controlled, and the method is easy to operate and high in practicability.
Owner:SHANGHAI JIAO TONG UNIV
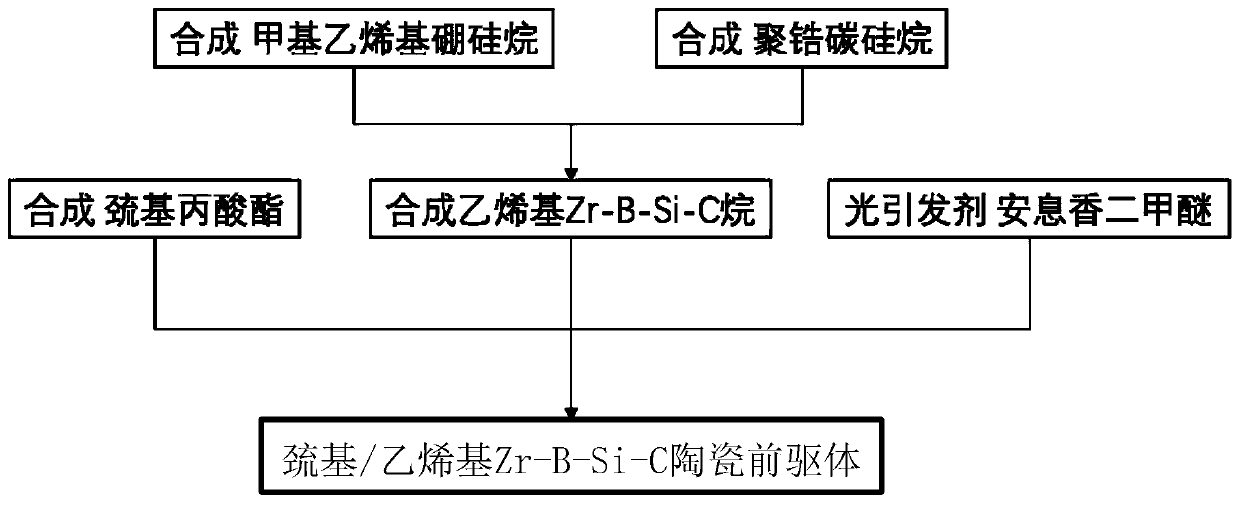
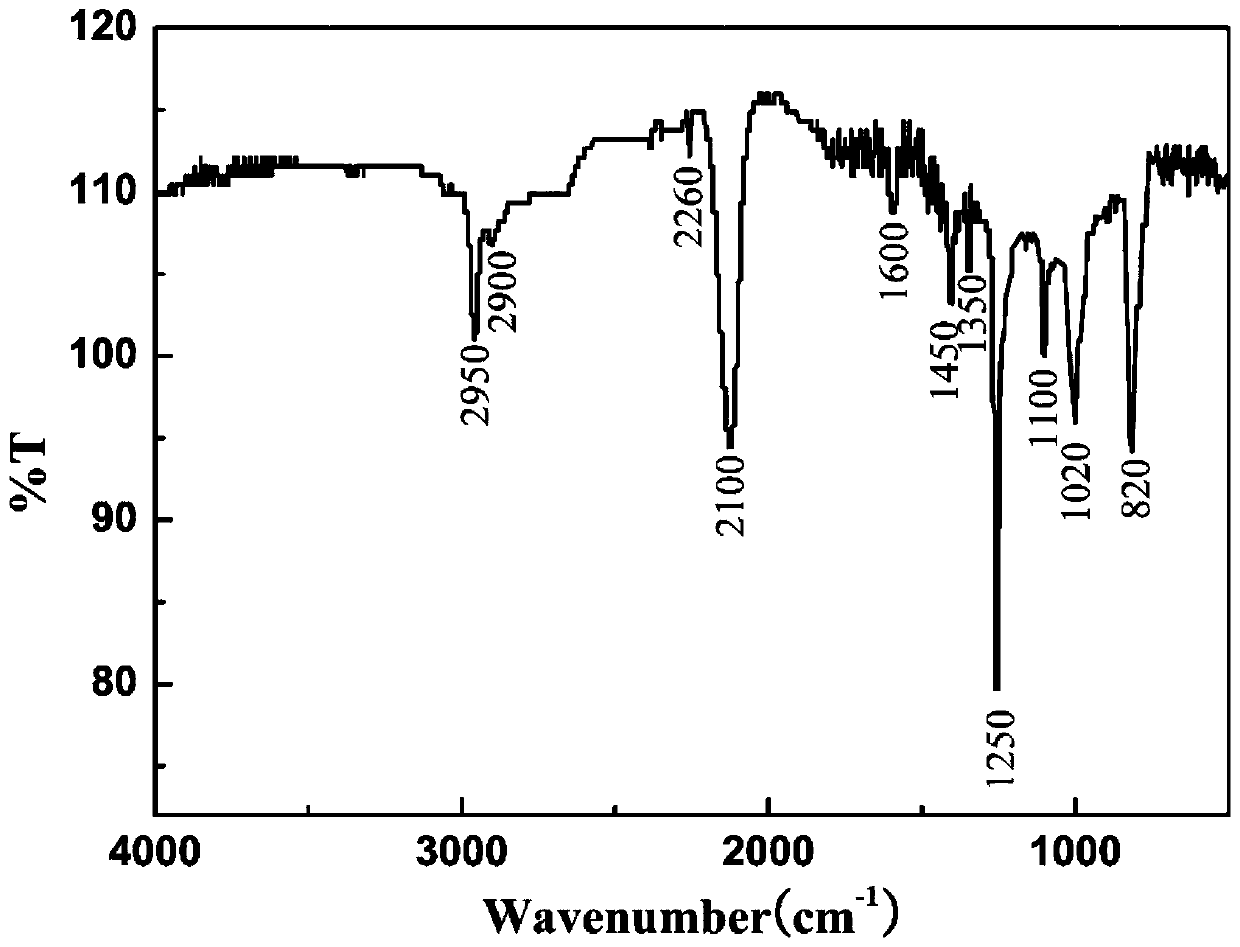
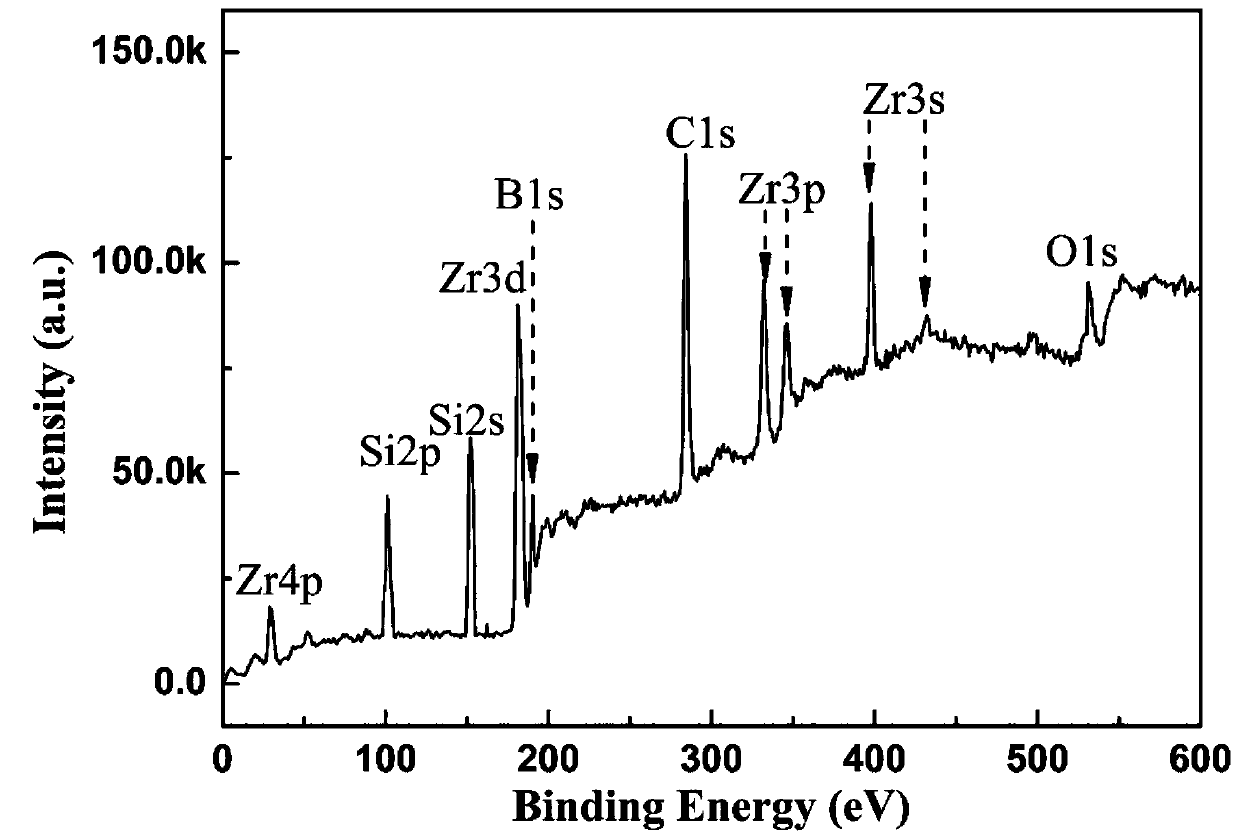
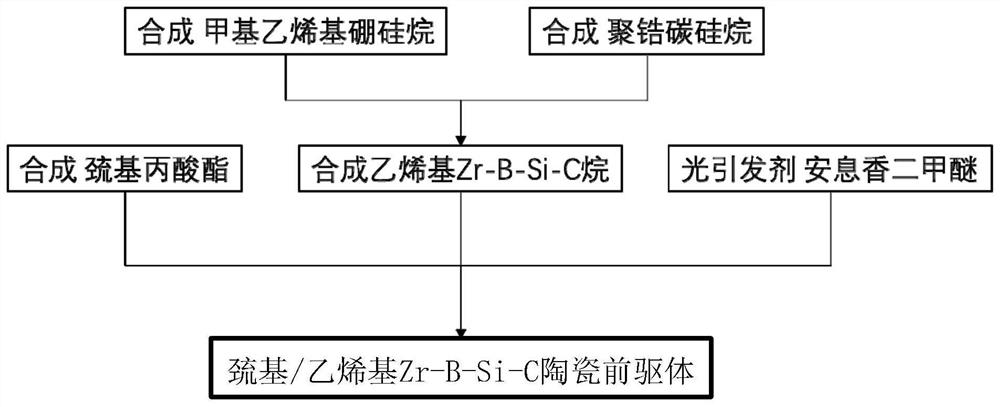
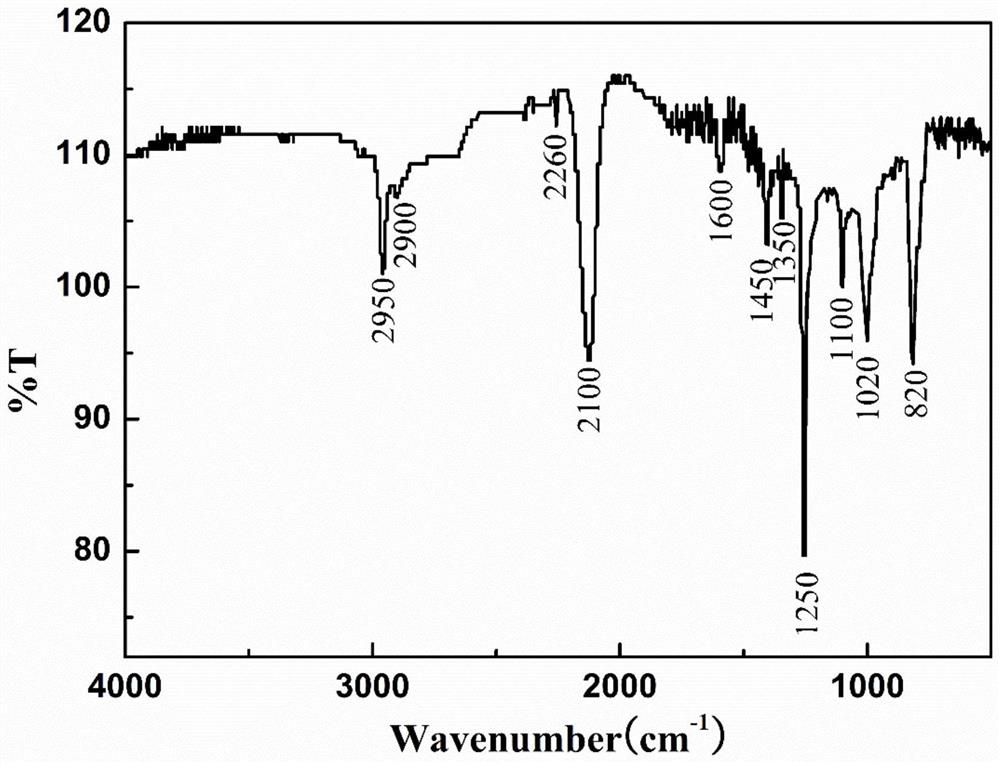
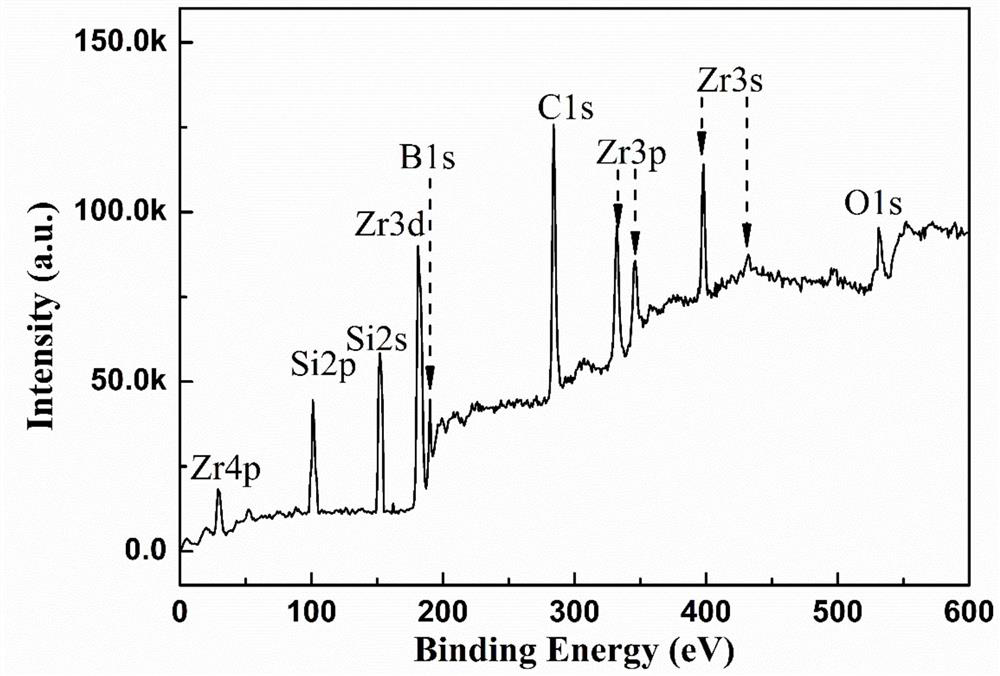
Light-sensitive Zr-B-Si-C ceramic precursor and in-situ preparation method thereof
The invention relates to a light-sensitive Zr-B-Si-C ceramic precursor and an in-situ preparation method thereof. The method comprises the following steps: uniformly mixing methylvinyldichlorosilane and a borane dimethyl sulfide complex, and then adding metal sodium for dechlorination to obtain methylvinyl borosilane; uniformly mixing chloromethyltrichlorosilane, methylchloromethyldichlorosilane and bis(cyclopentadienyl)zirconium dichloride, then adding metal magnesium to carry out a first heat preservation reaction, and then adding a reducing agent to carry out a second heat preservation reaction to obtain polyzirconocarbosilane; uniformly mixing methylvinyl borosilane and polyzirconocarbosilane to obtain vinyl Zr-B-Si-C alkane; and uniformly mixing the vinyl Zr-B-Si-C alkane with mercaptopropionate, and then adding a photoinitiator to initiate a polymerization reaction to prepare the ceramic precursor. The problems of high viscosity, high thermal stress, structural member mechanicalproperty attenuation and the like of a traditional photocuring system are solved, and a high-quality raw material is provided for photocuring 3D printing of an ultrahigh-temperature ceramic structuralmember.
Owner:XIAMEN UNIV OF TECH
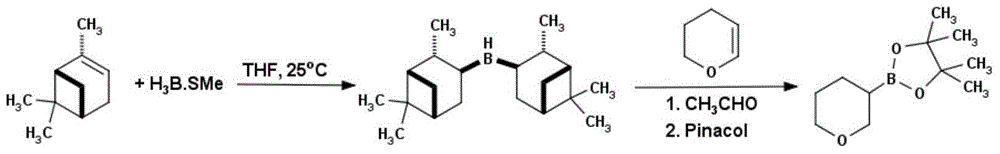
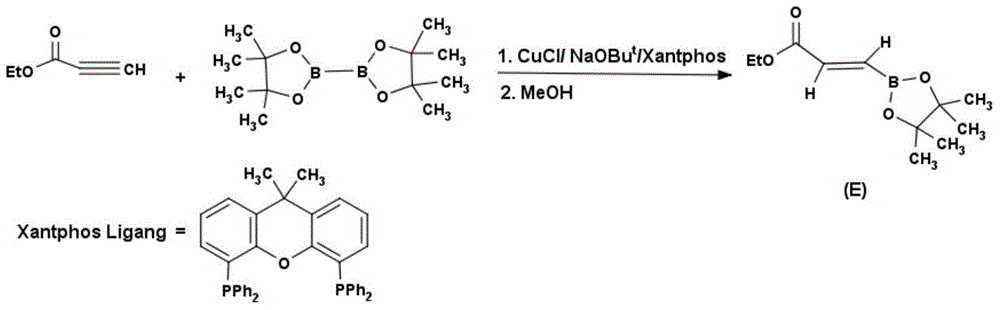
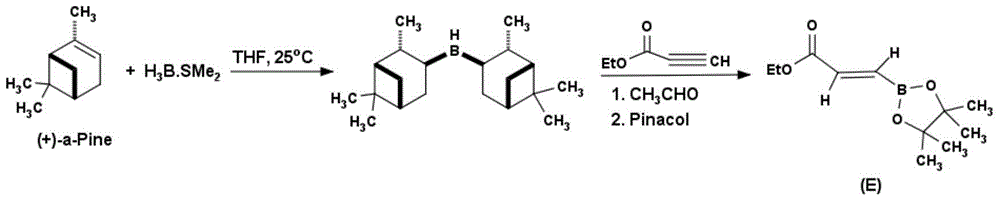
Method for preparing (E)-3-ethyl acrylate pinaborate
InactiveCN104610330ACheap and easy to getThe reaction steps are simpleGroup 3/13 element organic compoundsBorane dimethylsulfideRoom temperature
The invention discloses a method for preparing a medical intermediate (E)-3-ethyl acrylate pinaborate. The method specifically comprises the following steps: preparing di-alpha-pinoborane by using dextro-alpha-pinene and a borane-dimethyl sulfide complex as raw materials, then reacting di-alpha-pinoborane with ethyl propiolate at room temperature, then using anhydrous acetaldehyde for reduction to generate dimethyl borate and then reacting dimethyl borate with pinacol to generate (E)-3-ethyl acrylate pinaborate. The method has the obvious advantages that the reaction raw materials are easy to obtain; the reaction operation is simple; large-scale production is easy to achieve; the yield is high; the purity is good; the production cost is low.
Owner:成都安斯利生物医药有限公司
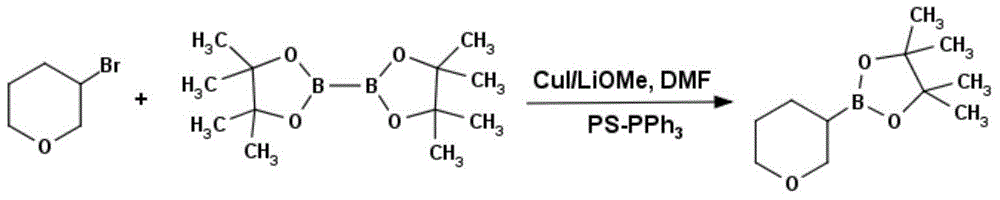
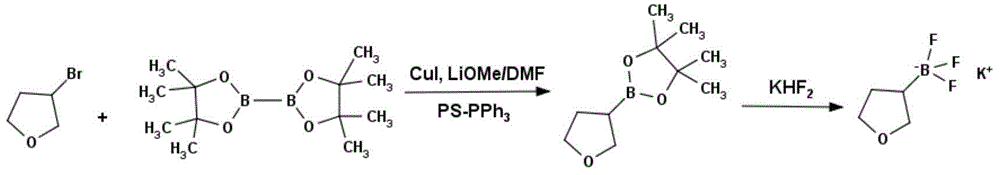
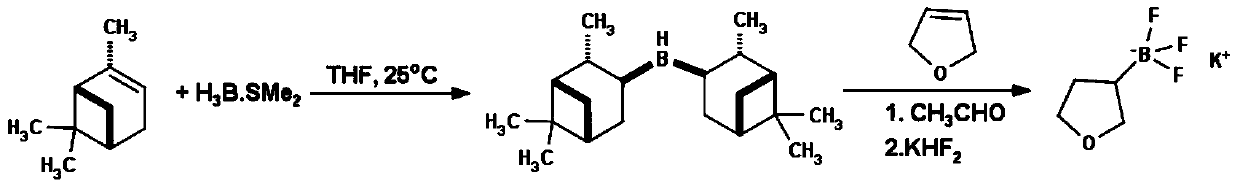
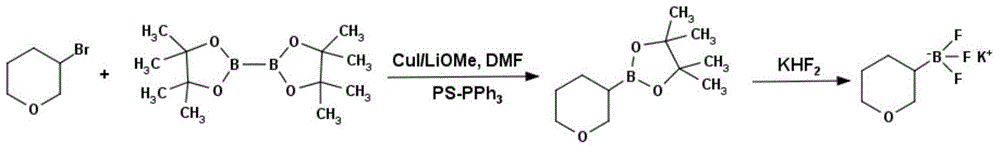
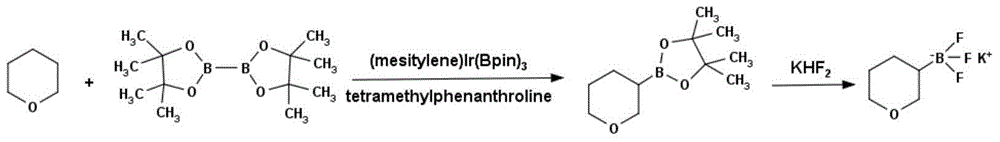
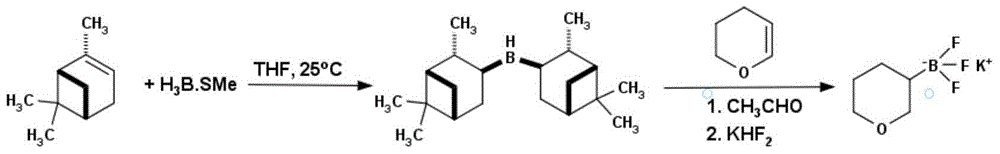
Method for preparing tetrahydropyran-3-potassium trifluoroborate
InactiveCN104610333ACheap and easy to getThe reaction steps are simpleGroup 3/13 element organic compoundsBorane dimethylsulfidePotassium
The invention discloses a new method for preparing tetrahydropyran-3-potassium trifluoroborate. The method specifically comprises the following steps: preparing di-alpha-pinoborane by using dextro-alpha-pinene and a borane-dimethyl sulfide complex as raw materials, then reacting di-alpha-pinoborane with 3,4-dihydropyran at room temperature, then using anhydrous acetaldehyde for reduction to generate dimethyl borate, without separating an intermediate, and subsequently reacting dimethyl borate with a saturated aqueous solution of KHF2 to generate the target product tetrahydropyran-3-potassium trifluoroborate. The method has the obvious advantages that the reaction raw materials are easy to obtain; the reaction operation is simple; large-scale production is easy to achieve; the yield is high; the purity is good; the production cost is low.
Owner:成都安斯利生物医药有限公司
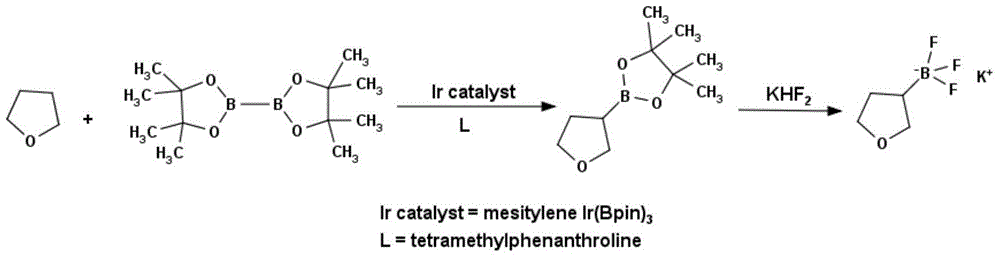
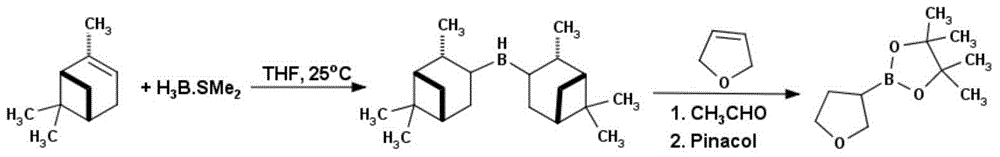
Method for preparing tetrahydrofuran-3-boric acid pinacol ester
InactiveCN104672261ASimple priceLow priceGroup 3/13 element organic compoundsBorane dimethylsulfideRoom temperature
Owner:成都安斯利生物医药有限公司
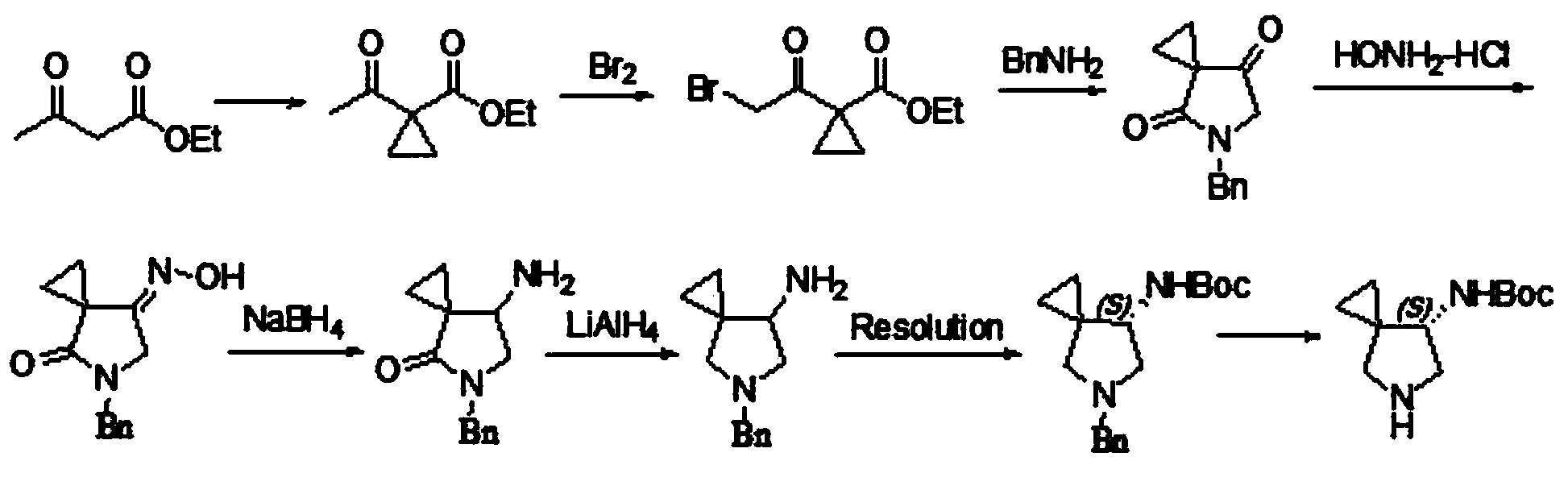
Synthesis method of tert-butyl-8-oxa-3, 11-diazaspiro [5.6] dodecane-3-formate
The invention relates to a synthesis method of tert-butyl-8-oxa-3, 11-diazaspiro [5.6] dodecane-3-formate, and mainly solves the technical problem that no suitable industrial synthesis method exists at present. The method comprises the following four steps: 1, reacting a compound 1 with ethyl bromoacetate to obtain a compound 2; 2, adding raney nickel into the compound 2 so as to obtain a compound3 through hydrogenation reduction; 3, cyclizing the compound 3 in ethanol by using sodium ethoxide to obtain a compound 4; and 4, reducing amide of the compound 4 in tetrahydrofuran by using a boranedimethyl sulfide complex to obtain a compound 5, with the reaction formula shown in the descriptions in the invention.
Owner:成都药明康德新药开发有限公司
Synthesis method for 2-(2-bromoethyl)benzoic acid methyl ester
InactiveCN102850221AOrganic compound preparationCarboxylic acid esters preparationBenzoic acidSynthesis methods
The present invention discloses a synthesis process for 2-(2-bromoethyl)benzoic acid methyl ester. According to the synthesis process, easily-available 2-formyl benzoic acid methylester is adopted as a raw material, and reacts with methyl triphenylphosphine hydroiodide in the presence of a strong base to obtain 2-methyl vinylbenzoate; the 2-methyl vinylbenzoate reacts with a borane dimethyl sulfide complex; 2-(2-hydroxyethyl)benzoic acid methyl ester is produced under conditions of sodium hydroxide and hydrogen peroxide; and finally the 2-(2-hydroxyethyl)benzoic acid methyl ester is converted into the 2-(2-bromoethyl)benzoic acid methyl ester under conditions of triphenylphosphine and carbon tetrabromide.
Owner:CGENETECH (SUZHOU CHINA) CO LTD
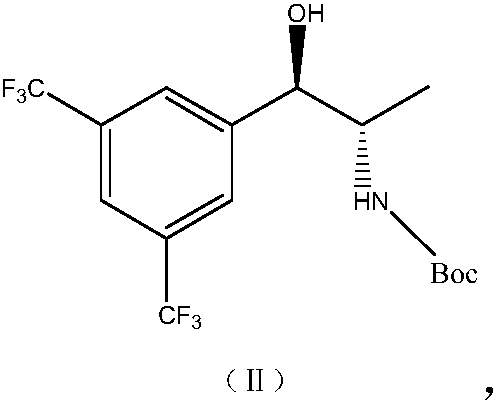
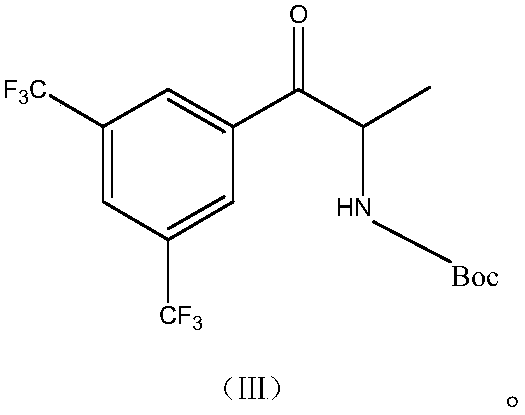
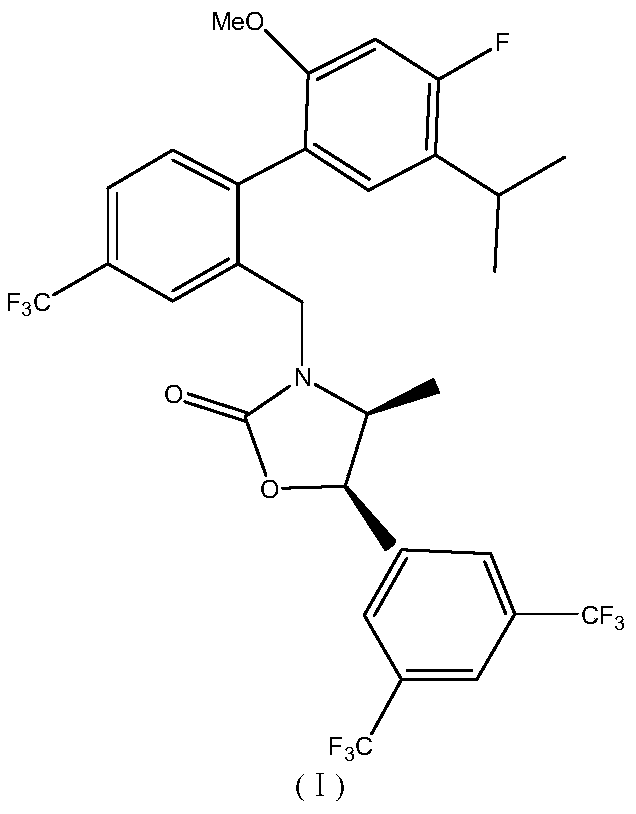
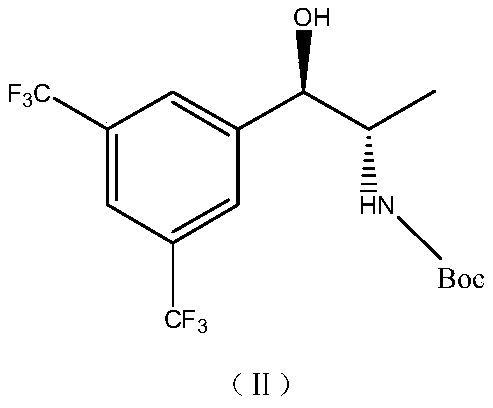
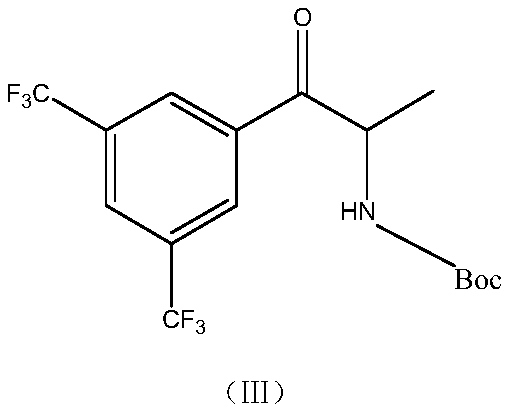
Synthesis method of anacetrapib chiral intermediate
ActiveCN107793330AReduce manufacturing costHigh selectivityCarbamic acid derivatives preparationOrganic compound preparationPropanolSynthesis methods
The present invention relates to a method for synthesizing an anacetrapib intermediate (1R,2S)-1-(3,5-bis(trifluoromethyl)phenyl)-2-Boc-amino-propanol. The method comprises: adding (R)-2-methyl-CBS-oxazaborolidine and a borane-dimethyl sulfide complex to 1-(3,5-bis(trifluoromethyl)phenyl)-2-Boc-amino-acetone in a certain solvent at a certain temperature, and carrying out a reducing reaction. According to the present invention, the chiral catalytic reaction does not use the expensive metallic ruthenium catalyst, the catalytic selectivity is high, the reaction condition is mild, the product is single, the obtained intermediate has the high optical purity and the high yield, the production cost is low, and the method is environment is environmentally friendly, and is suitable for Industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
Dimethyl sulfide-containing tail gas absorption apparatus and dimethyl sulfide-containing tail gas absorption method
InactiveCN106362565AReduce contentLow costDispersed particle separationToxic gasBorane dimethylsulfide
The invention discloses a dimethyl sulfide-containing tail gas absorption method, wherein sulfur-containing substances are subjected to oxidation degradation with an economic oxidizing agent. The method specifically comprises: 1, adding a degradation oxidizing agent into a gas scrubber; 2, introducing borane dimethyl sulfide reaction tail gas or dimethyl sulfide tail gas into the gas scrubber of the step 1; and 3, in the gas scrubber, carrying out an oxidation degradation reaction on the borane dimethyl sulfide reaction tail gas or dimethyl sulfide tail gas and the degradation oxidizing agent to achieve the emission standard. The present invention further discloses a dimethyl sulfide-containing tail gas absorption apparatus. According to the present invention, with the method and the apparatus, the odor and toxic gas emission is controlled and the environment is protected while the raw material consumption is reduced so as to achieve the sustainable development.
Owner:上海福乐医药科技有限公司
Synthesis method of Mirabegron
The invention discloses a synthesis method of Mirabegron. The synthesis method comprises the following processing steps of S1, under the room-temperature condition, adding R-2 (amino-N-methylbenzylamine) benzyl alcohol,2-(2-Aminothiazole-5-based)-N-[4-(2-Chloro-Ethyl)-Phenyl]-Acetamide and triethylamine to a solvent, and performing reflowing for 8h; S2, after the reaction is finished, pumping reaction liquid into water, and performing filtering to obtain the Mirabegron. In the step S1, the solvent is one or more of methanol, ethanol, propanol, dichloromethane, tri- chloromethane, acetone, butanone and toluene. An organic solvent which has stimulability is not used, a borane-methyl sulfide complex having high risk is not used, high temperature and high pressure are not adopted, the reactionprocess is green and environmental-friendly, clean production is realized, the cost is reduced, and the synthesis method has good market competitiveness.
Owner:SUZHOU UUGENE BIOPHARMA
A photosensitive zr-b-si-c ceramic precursor and its in-situ preparation method
Owner:XIAMEN UNIV OF TECH
Synthetic method of triphenoxyboroxine
InactiveCN108586510AHigh yieldHigh puritySecondary cellsGroup 3/13 element organic compoundsBorane dimethylsulfideAcid value
A synthetic method of triphenoxyboroxine belongs to the technical field of battery electrolyte and includes: subjecting borane-dimethylsulfide complex as a raw material to reaction with phenol to obtain triphenoxyboroxine, adding borane-dimethylsulfide complex into a Schlenk reaction flask, adding THF (tetrahydrofuran), mixing, adding phenol and water into the mixture, heating to allow reacting under 70-80 DEG C and 1-5 atm for 1-1.5 h, allowing reacting under 70-80 DEG C and 8-120 Pa for 30-40 min, allowing reacting under 3-5 Pa and 80-100 DEG C for 20-30 min, concentrating and drying, and cooling to room temperature to obtain triphenoxyboroxine. The synthetic method has the advantages that preparation is simple, a preparation technique is good in cleanliness and environmental friendliness, the preparation process is mild and stable, and prepared triphenoxyboroxine has high yield, high purity, low water content and low acid value.
Owner:SHIJIAZHUANG SAN TAI CHEM CO LTD
The preparation method of chiral S or r-3-ethoxy-4-methoxy-α-[(methylsulfonyl) methyl] benzyl alcohol
InactiveCN105461602BShort reaction timeReduce waste disposalCarboxylic acid nitrile preparationOrganic compound preparationMethyl groupBENZYL ALCOHOL/WATER
The invention relates to a preparation method of chiral S / R-3-ethoxy-4-methoxy-alpha[(methylsulfonyl)methyl] benzyl alcohol. The preparation method comprises the following steps: 3-hydroxy-4-methoxybenzaldehyde is taken as a starting material and reacts with hydroxylammonium hydrochloride to produce 3-hydroxy-4-methoxybenzonitrile; 3-hydroxy-4-methoxybenzonitrile reacts with bromoethane to produce 3-ethoxy-4-methoxybenzonitrile; 3-ethoxy-4-methoxybenzonitrile reacts with dimethyl sulfone under the action of n-butyllithium, a product is hydrolyzed in an aqueous hydrochloric acid solution, and 1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethanone is obtained; finally, S-(-)-alpha,alpha-diphenyl-2-pyrrolidinemethanol or R-(+)-alpha,alpha-diphenyl-2-pyrrolidinemethanol is taken as a chiral catalyst, a borane dimethyl sulfide solution is taken as a reducing agent, carbonyl is reduced, and a product is obtained. The reaction conditions are mild, the product yield is higher, the technology level is increased, the operability is improved, and large-scale industrial production is facilitated.
Owner:DONGHUA UNIV
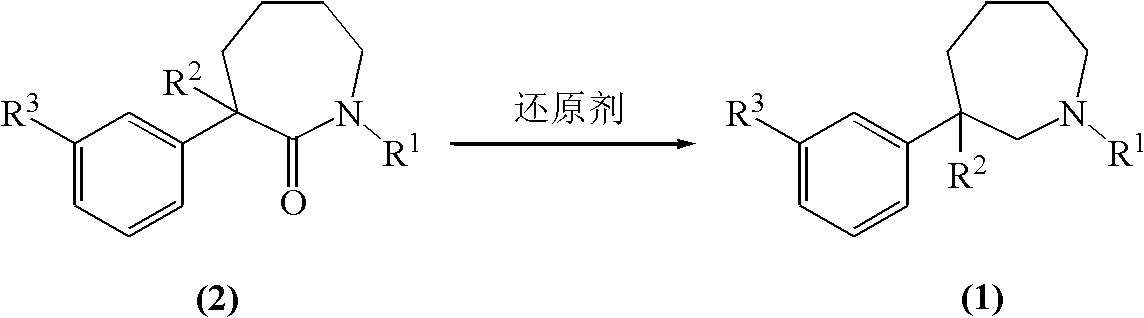
Method for preparing meptazinol and analogs thereof
The invention relates to a method for preparing meptazinol and analogs thereof, in particular to a method for preparing a compound shown in the general formula (1) from a compound shown in the general formula (2). Reducing agents are selected from one kind of or several kinds of materials in dihydro (2-methoxyethoxy) sodium aluminate (vitride solution), sodium borohydride, boron triflouride-aether, boron triflouride aether-sodium borohydride, boron triflouride and borane-methyl sulfide and is preferably the vitride solution. The reducing agents used in the method are stable in the air, in addition, the material addition is easy, and the quenching process can be completed in a shorter time, so the operation requirement is low, and the defect that the fire catching and the explosion are easy to occur in the traditional tetrahydro aluminum lithium reduction method is overcome.
Owner:CHINA RESOURCES DOUBLE CRANE PHARMA COMPANY
A kind of synthetic method of anseltrapib chiral intermediate
ActiveCN107793330BReduce manufacturing costHigh selectivityCarbamic acid derivatives preparationOrganic compound preparationPtru catalystAnacetrapib
Owner:LUNAN PHARMA GROUP CORPORATION
A kind of preparation method of Apremilast
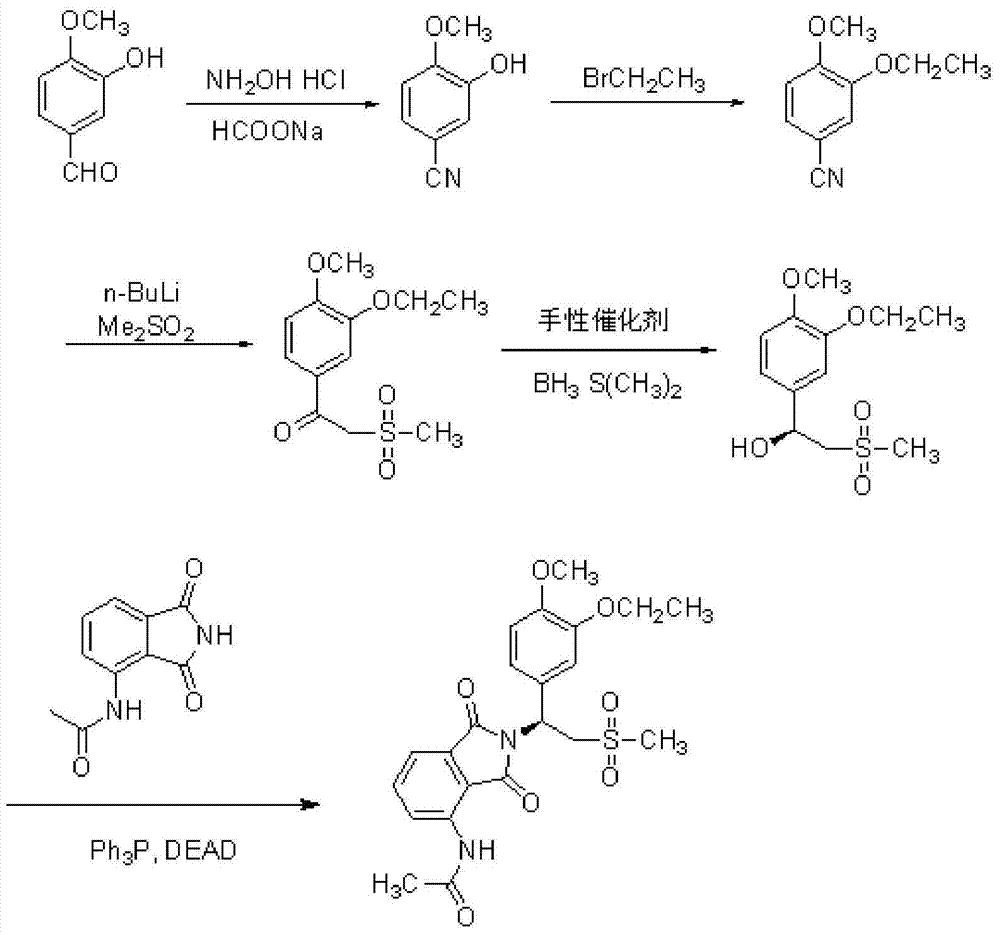
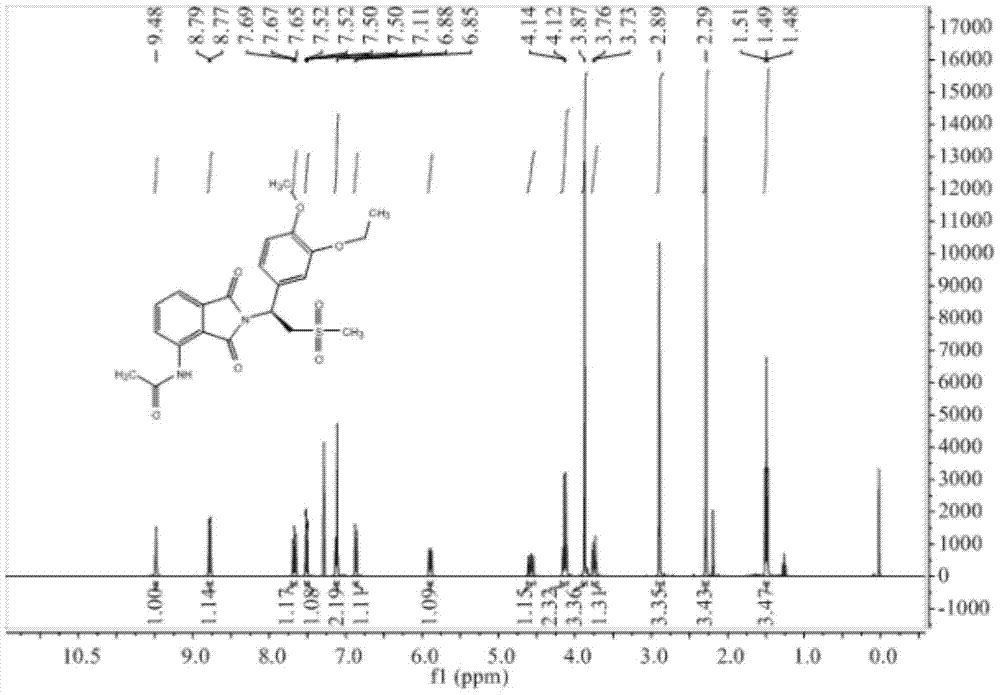
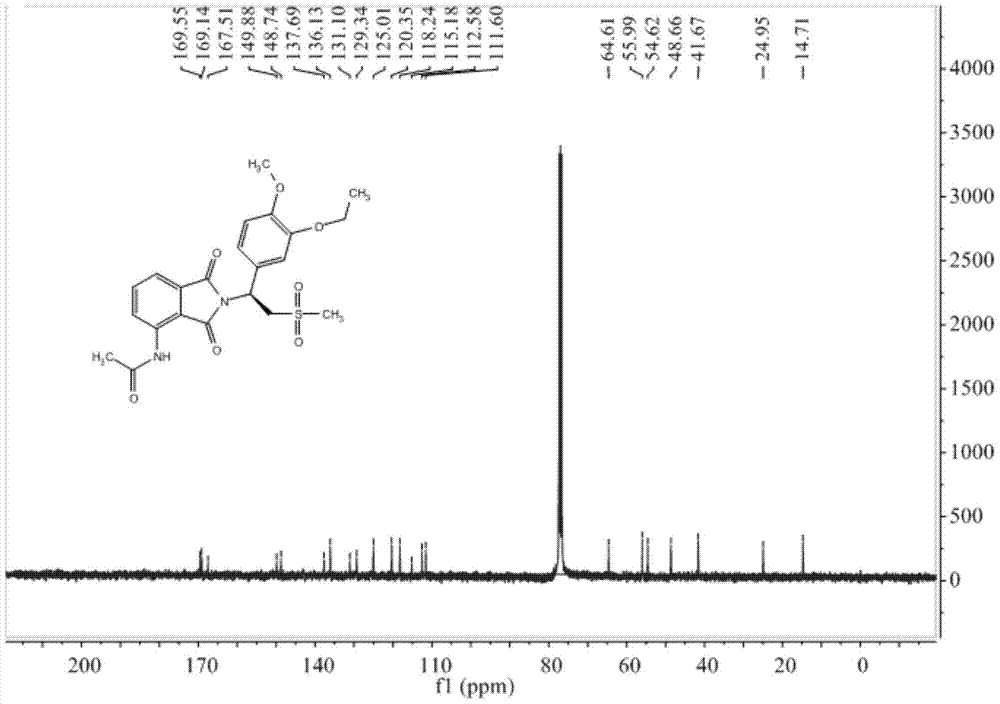
InactiveCN105330586BAvoid splittingReduce waste disposalOrganic chemistryHydroxylamine HydrochlorideBromoethane
The invention relates to a preparation method of Apremilast, comprising the following steps: by taking 3-hydroxy-4-methoxybenzaldehyde as a staring material, reacting with hydroxylamine hydrochloride to obtain 3-hydroxy-4-methoxy cyanobenzene, reacting with bromoethane to obtain 3-ethyoxyl-4-methoxy cyanobenzene, and then reacting with dimethyl sulfone under the action of n-butyllithium, and hydrolyzing in aqueous hydrochloric acid solution to obtain 1-(3-ethyoxyl-4-methoxyphenyl)-2-(mesyl)ketol); finally by taking S-(-)-alpha, alpha-diphenyl-2-pyrrolidine methanol as a chiral catalyst and taking borane dimethyl sulfide complex solution as a reductant, obtaining chirality S-3-ethyoxyl-4-methoxy group-alpha[(mesyl)benzyl alcohol], and then reacting with 3-acetamido-phthalimide under the action of triphenylphosphine and diethyl azodicarboxylate, thus obtaining the Apremilast. According to the preparation method, the process is effectively simplified, the reaction conditions are mild, the product yield and purity are relatively high, and large-scale industrial production is benefited.
Owner:DONGHUA UNIV
Preparation method of 2,2,2-trifluoro-N-[(1S,4S)-4-hydroxy tetrahydronaphthalene-1-yl]-acetamide
InactiveCN108558695AEmission reductionEasy to operateOrganic compound preparationCarboxylic acid amides preparationManufacturing cost reductionBorane dimethylsulfide
The invention discloses a preparation method of 2,2,2-trifluoro-N-[(1S,4S)-4-hydroxy-1,2,3,4-tetrahydronaphthalene-1-yl]-acetamide. The preparation method comprises the following steps: performing asymmetric reduction on 2,2,2-trifluoro-N-[(S)-4-carbonyl-1,2,3,4-tetrahydronaphthalene-1-yl]-acetamide under the action of chiral oxazaborolidine derivative and borane-methyl sulfide to obtain a 2,2,2-trifluoro-N-[(1S,4S)-4-hydroxy-1,2,3,4-tetrahydronaphthalene-1-yl]-acetamide reaction solution; after the reaction ends, adding diluted hydrochloric acid for quenching, filtering, pulping with water, and carrying out solid drying to obtain a pure product, wherein the yield is 95.0% or higher, the purity is 99.0% or higher, and the ee% value is 99.0% or higher. According to the preparation method disclosed by the invention, the asymmetric reduction is carried out under the combined action of chiral oxazaborolidine derivative and borane-methyl sulfide, the reaction is simple in operation, the preparation period is shortened, the manufacturing cost is reduced, the generation of solid waste and solid water is greatly reduced, the preparation method is beneficial for environmental protection, and the investment of an enterprise for the disposal of waste is reduced.
Owner:浙江华贝药业有限责任公司
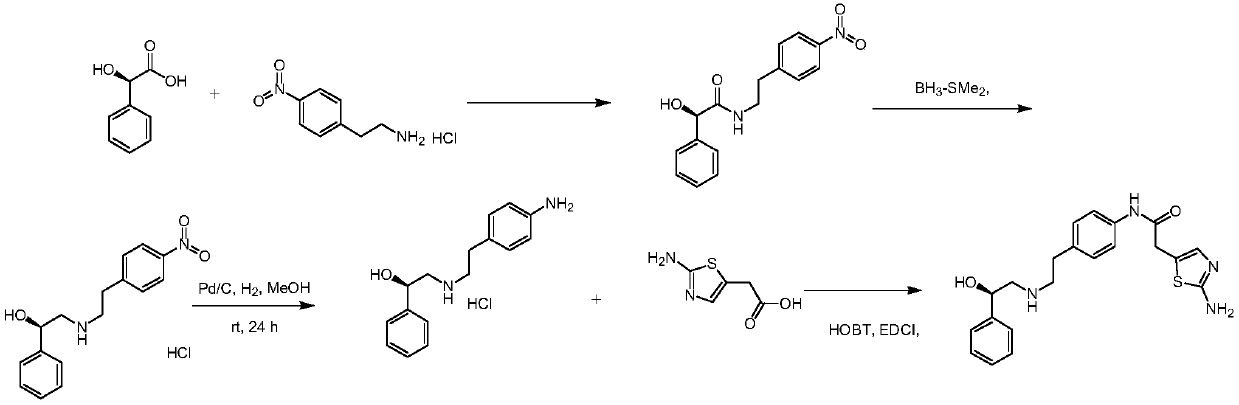
Synthesis method of (1R)-2-[[[2-(4-aminophenyl) ethyl] amino] methyl] benzyl alcohol
ActiveCN111004135AMild responseEasy to operateOrganic compound preparationOrganic chemistry methodsSolubilityEthyl group
The invention discloses a synthesis method of (1R)-2-[[[2-(4-aminophenyl) ethyl] amino] methyl] benzyl alcohol, which comprises the following processing steps: S1, adding R-2 (aminomethyl) benzyl alcohol, 4-(2-chloroethyl) aniline and triethylamine into a solvent at room temperature, and mixing; S2, after heating to 60-65 DEG C, stirring and refluxing for 1-48h; S3, pumping an obtained reaction liquid into water while the reaction liquid is hot; cooling and filtering to obtain a white solid (1R)-2-[[[2-(4-aminophenyl) ethyl] amino] methyl] benzyl alcohol; wherein the molar ratio of R-2 (aminomethyl) benzyl alcohol to 4-(2-chloroethyl) aniline in the step S1 is 1: (1-20), the solubility of 4-(2-chloroethyl) aniline in the solvent in the step S1 is 40%-60%, and the use amount of 4-(2-chloroethyl) aniline is 1-1.5 times of the mass amount of R-2 (aminomethyl) benzyl alcohol. The method has the advantages of mild reaction, simple operation, and green and environment-friendly reaction process; no irritant organic solvent is adopted; dangerous borane dimethyl sulfide is not adopted; high temperature and high pressure are not adopted; and clean production is realized.
Owner:SUZHOU UUGENE BIOPHARMA
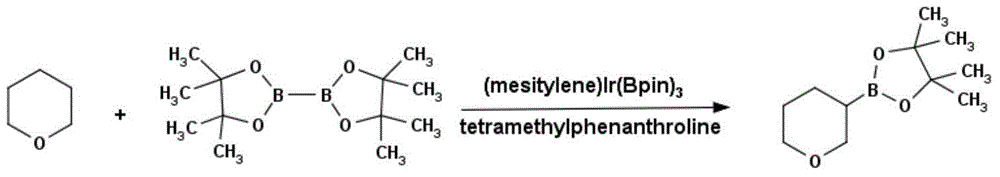
Method for preparing tetrahydropyrane-3-boronic acid pinacol ester
InactiveCN104592278ACheap and easy to getThe reaction steps are simpleGroup 3/13 element organic compoundsBorane dimethylsulfideBoronic acid
The invention discloses a novel method for preparing tetrahydropyrane-3-boronic acid pinacol ester. The new method specifically comprises the following steps: taking a dextrorotation alpha pinene and dimethyl sulfide borane complex compound as raw materials, preparing di-alpha pinene borane, then reacting with 3,4-dihydropyran at a room temperature, reducing with anhydrous acetaldehyde to generate dimethyl borate, needing no intermediate separation and successively reacting with pinacol to generate a target product, namely the tetrahydropyrane-3-boronic acid pinacol ester. The method has the obvious advantages of high easiness in obtaining of reaction raw materials, high simplicity in reaction operation, high easiness in realization of mass production, high yield, good purity and low production cost.
Owner:成都安斯利生物医药有限公司
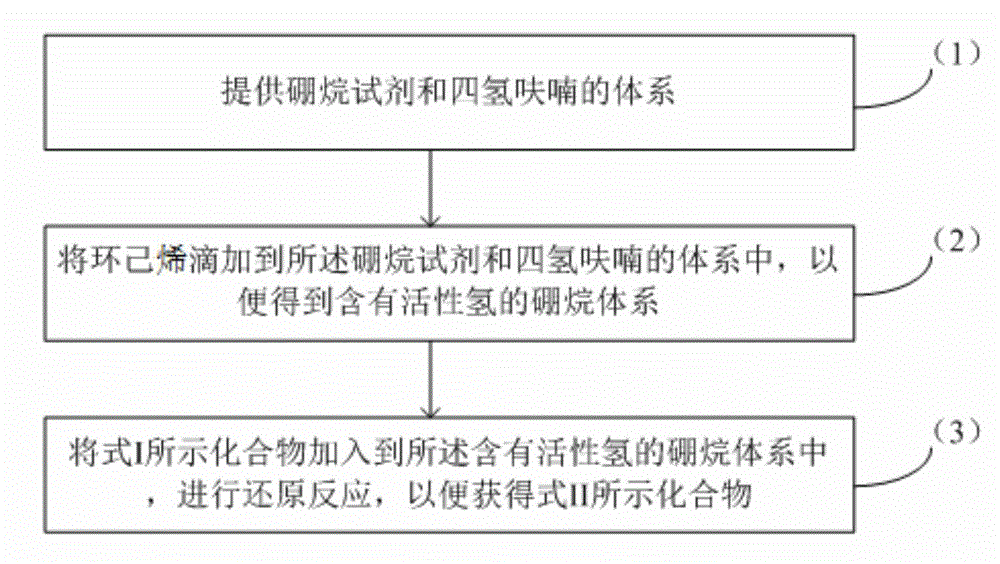
Method for reducing diyne compounds
ActiveCN104130091AEfficiently obtainedImprove efficiencyOrganic reductionOrganic compound preparationHydrogenCyclohexene
The invention provides a method for reducing diyne compounds. The method for reducing the compounds represented by a formula I comprises the following steps: (1) a system of a borane reagent and tetrahydrofuran is provided, wherein the borane reagent is a borane dimethylsulfide solvent; (2) cyclohexene is dropped into the system of borane reagent and tetrahydrofuran, such that a borane system comprising active hydrogen is obtained; (3) the compound represented by the formula I is added into the borane system comprising active hydrogen, and a reduction reaction is carried out, such that a compound represented by a formula II can be obtained. With the method, diyne compounds can be effectively reduced into diene compounds. In the entire process of the method, reaction materials are easy to obtain; reaction post-treatment is simple; industrial three-waste treatment is easy; and target product yield is high. The method is suitable for industrialized productions.
Owner:WATERSTONE PHARMA WUHAN
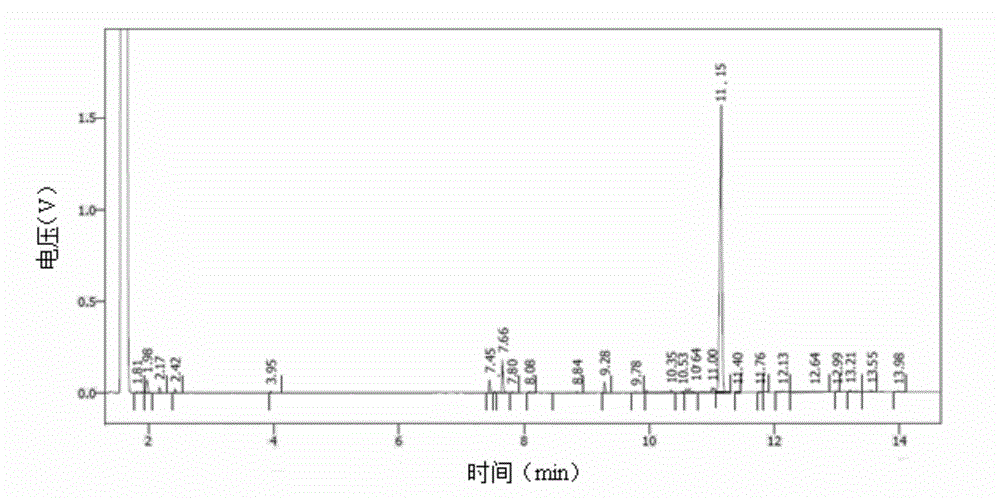
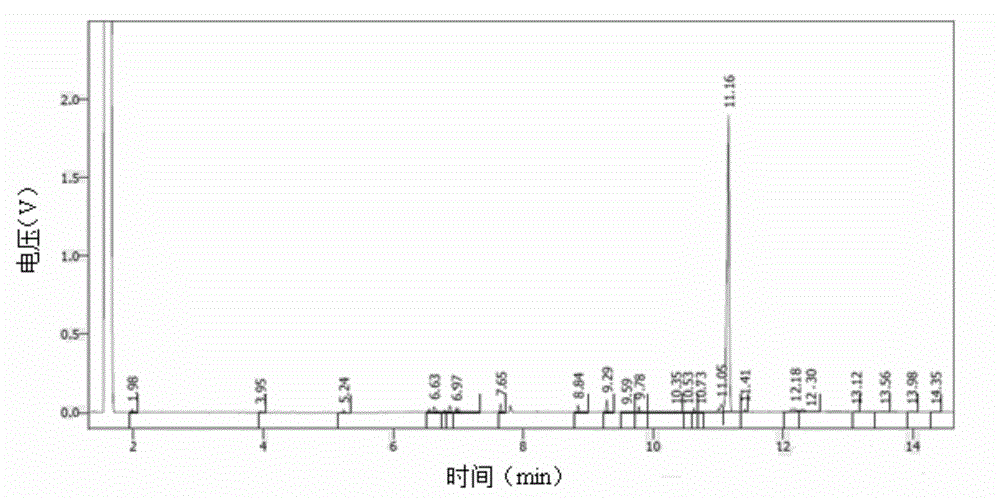
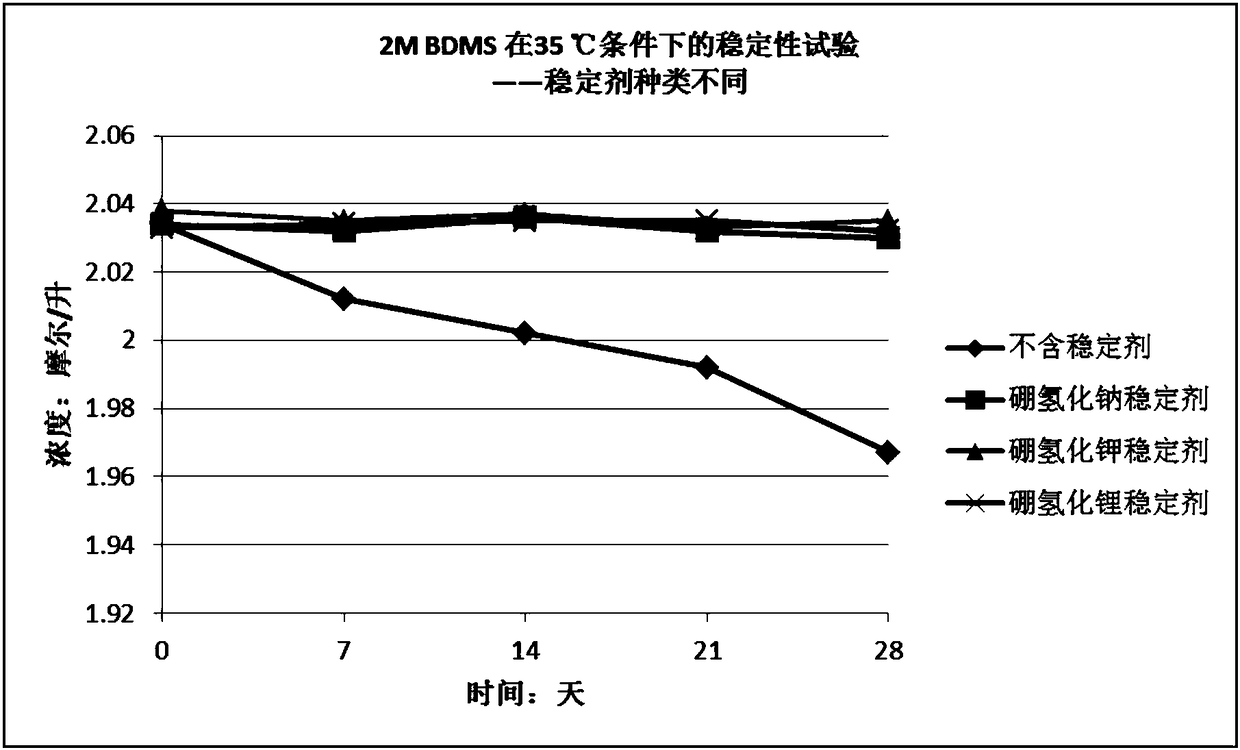
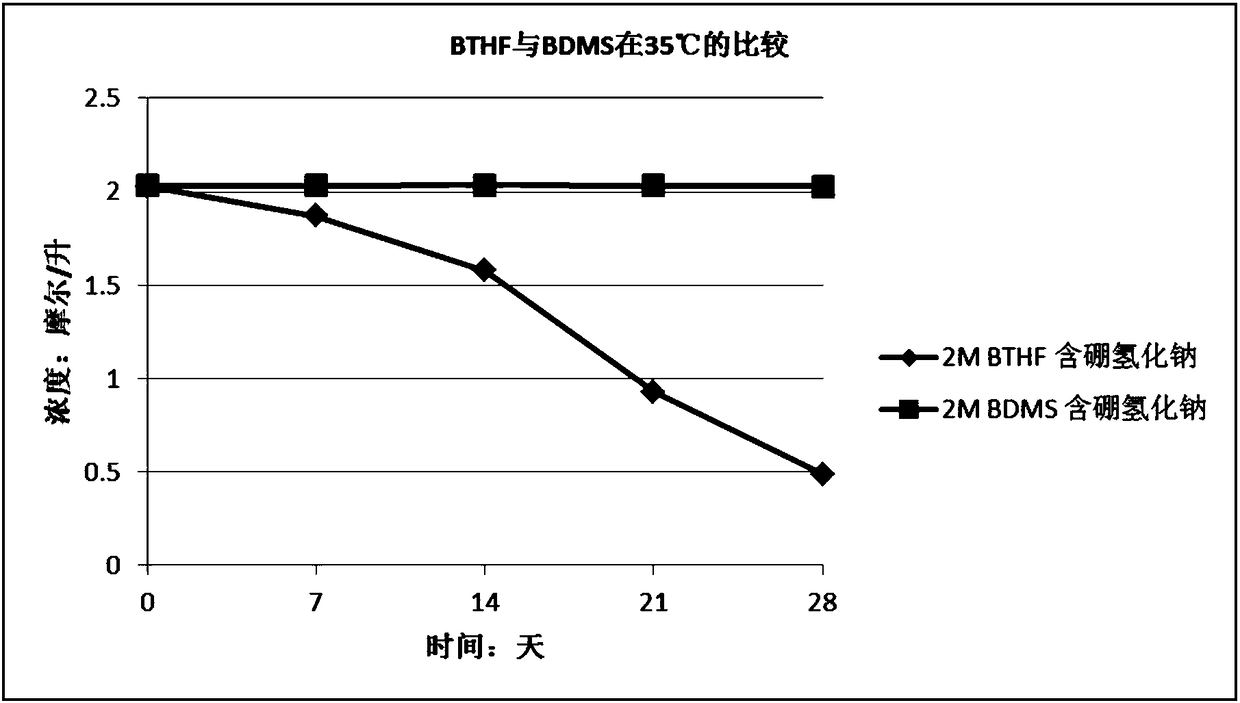
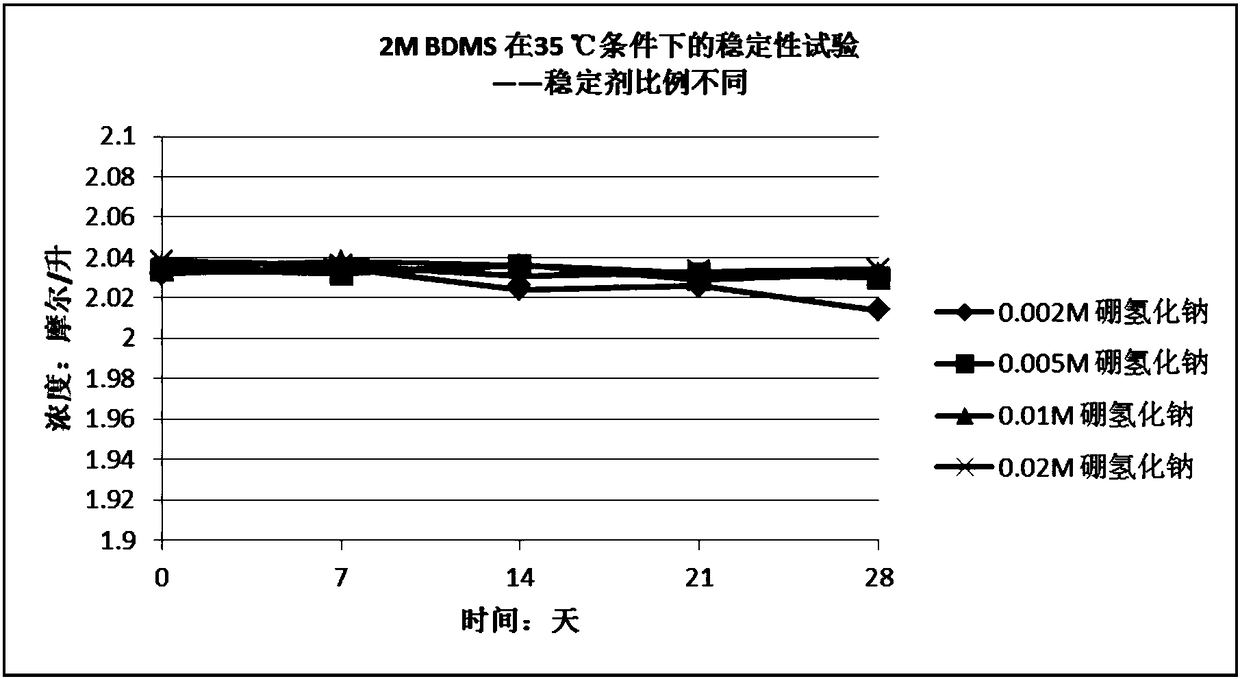
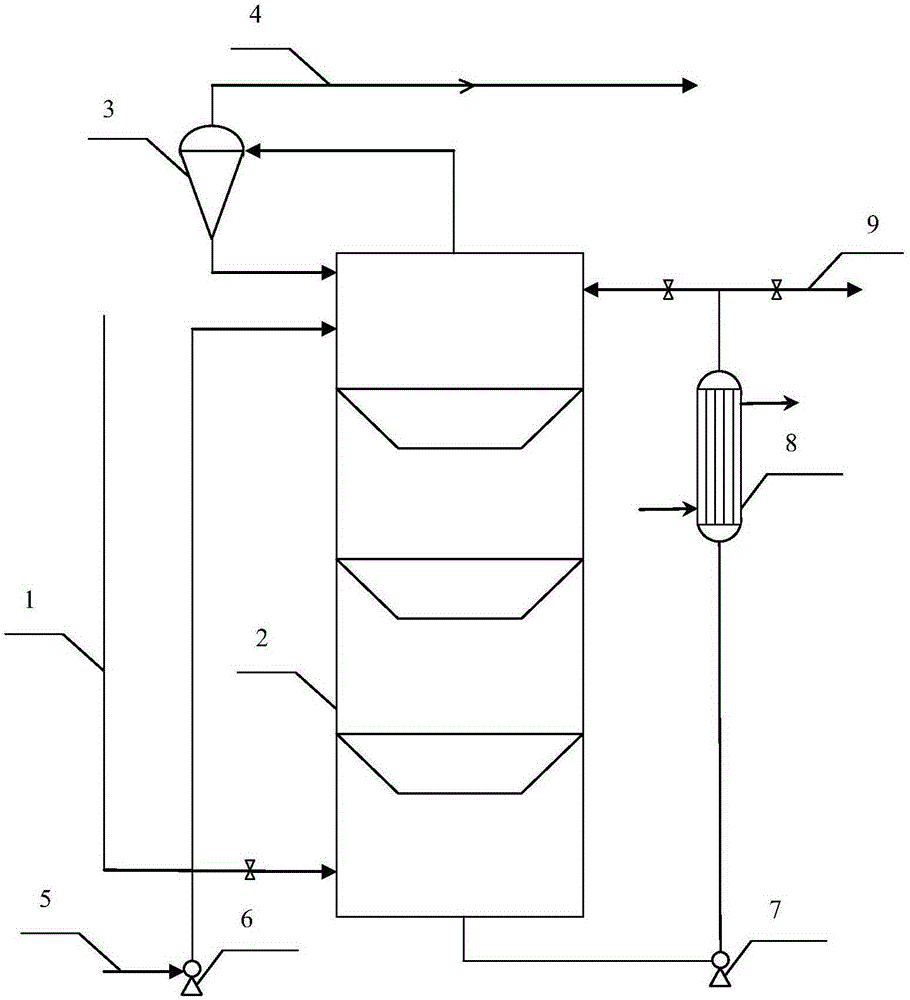
Stabilizer-containing borane reagent combination solution, and preparation method and use thereof
ActiveCN108059579AIncrease concentrationImprove thermal stabilityOrganic compound preparationHydroxy group formation/introductionHigh concentrationBorane dimethylsulfide
The invention provides a stabilizer-containing borane reagent combination solution. The borane reagent combination solution comprises a borane-dimethyl sulfide complex, tetrahydrofuran and a stabilizer, wherein the concentration of the borane-dimethyl sulfide complex in tetrahydrofuran is 1-10 mol / L, and a molar ratio of the borane-dimethyl sulfide complex to the stabilizer is 100:1 to 1000:1. Thecombination solution has the advantages of high concentration, good thermal stability, realization of high-efficiency utilization of a reaction container, and saving of the tetrahydrofuran reagent toreduce the cost; and the combination solution achieves a good reaction enantioselectivity and a high enantiomeric excess value (% ee) of a product when applied to Corey asymmetric reduction.
Owner:上海福乐医药科技有限公司
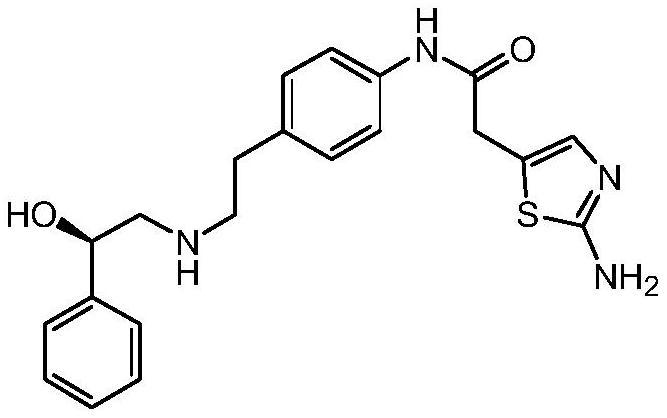
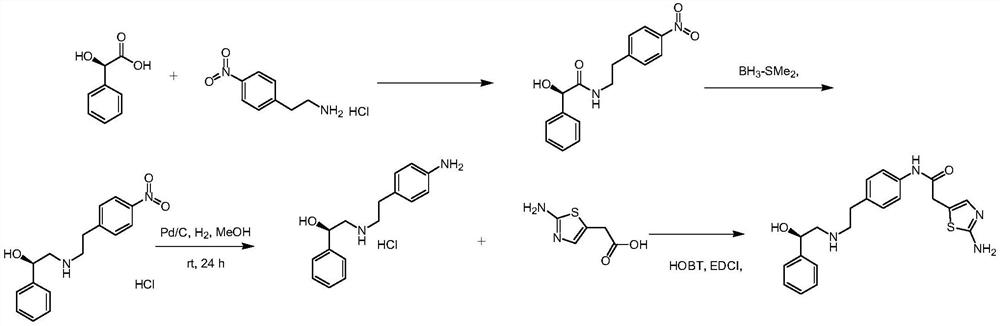
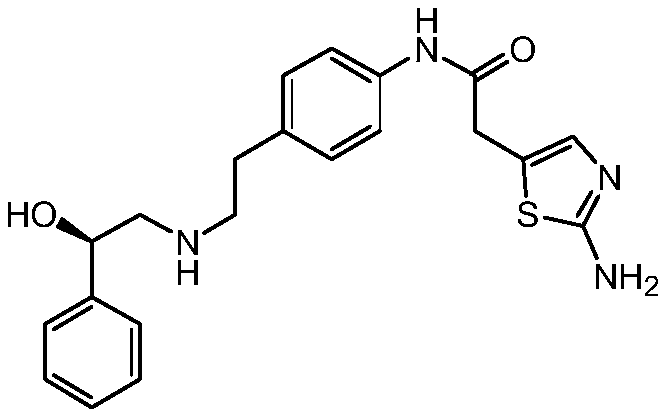
The synthetic method of mirabegron
The invention discloses a synthesis method of Mirabegron, which comprises the following processing steps: S1. Under room temperature conditions, R-2 (aminomethyl) benzyl alcohol, 2-(2-aminothiazole-5-yl)- N-[4-(2-chloro-ethyl)-phenyl]-acetamide and triethylamine were added to the solvent and refluxed for 8h; S2, after the reaction was completed, the reaction solution was pumped into water, filtered to obtain Mirabegron . The solvent in the step S1 is one or more of methanol, ethanol, propanol, dichloromethane, chloroform, acetone, butanone, and toluene. The present invention not only does not use irritating organic solvents, but also does not use dangerous borane dimethyl sulfide and high temperature and high pressure, not only the reaction process is green and environmentally friendly, clean production is realized, but also the cost is reduced, which has better market competition force.
Owner:SUZHOU UUGENE BIOPHARMA
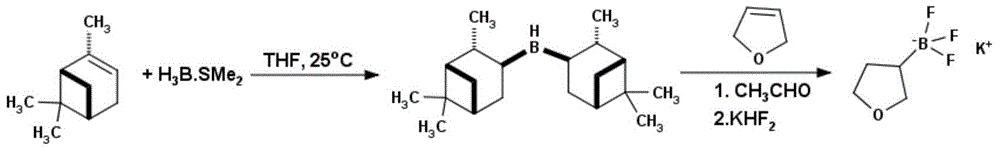
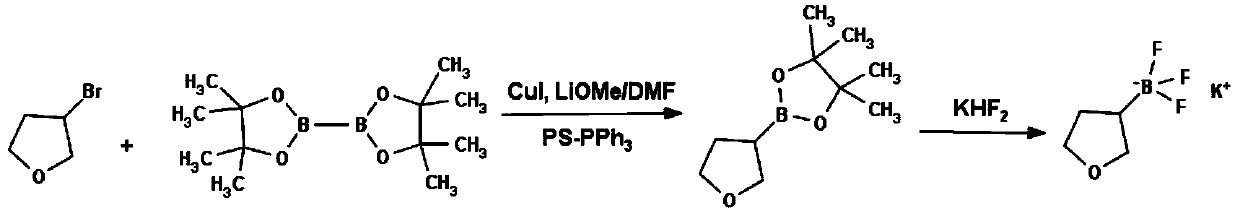
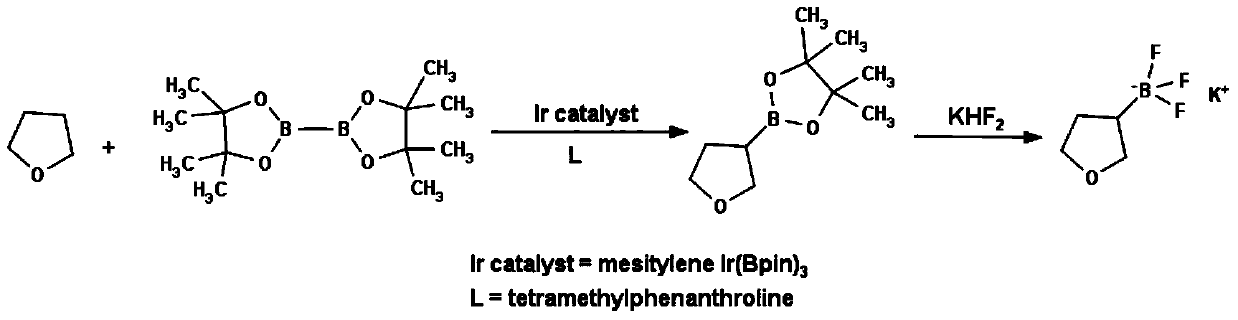
Method for preparing tetrahydrofuran-3-potassium trifluoroborate
InactiveCN104610332ASimple priceLow priceGroup 3/13 element organic compoundsBorane dimethylsulfideRoom temperature
The invention discloses a new method for preparing tetrahydrofuran-3-potassium trifluoroborate. The new method specifically comprises the following steps: preparing di-alpha-pinoborane by using dextro-alpha-pinene and a borane-dimethyl sulfide complex as raw materials, then reacting di-alpha-pinoborane with 2,5-dihydrofuran at room temperature, then using anhydrous acetaldehyde for reduction to generate dimethyl borate, without separating an intermediate, and subsequently reacting dimethyl borate with a saturated aqueous solution of KHF2 to generate the target product tetrahydrofuran-3-potassium trifluoroborate. The method has the obvious advantages that the reaction raw materials are easy to obtain; the reaction operation is simple; large-scale production is easy to achieve; the yield is high; the purity is good; the production cost is low.
Owner:成都安斯利生物医药有限公司
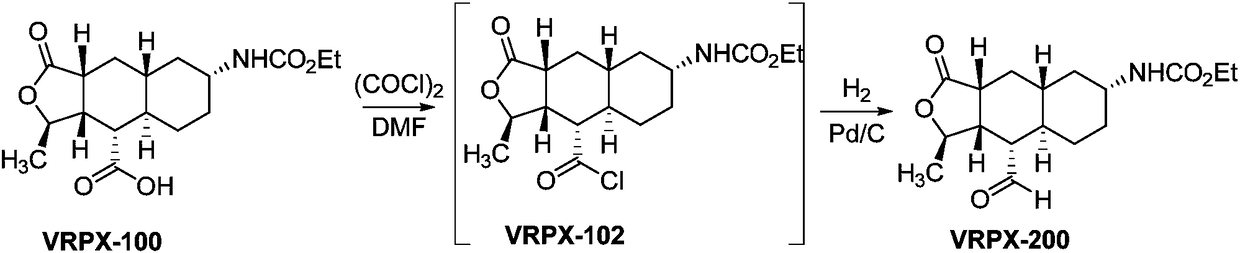
Method for preparing vorapaxar intermediate
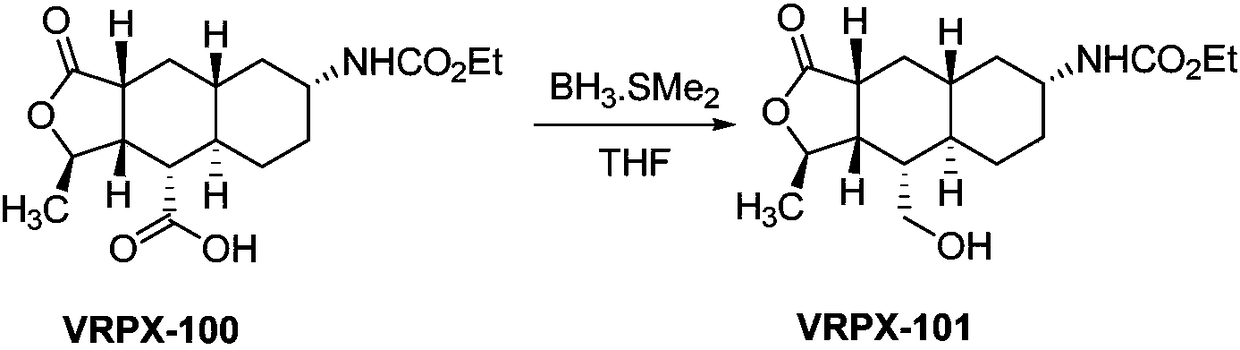
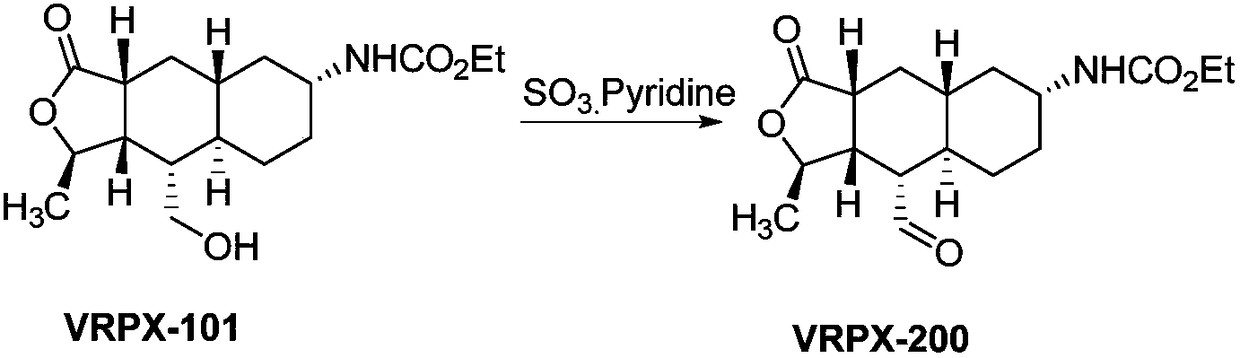
InactiveCN108947947AHigh reaction yieldReduce the difficulty of production operationsOrganic chemistryAlcoholBorane dimethylsulfide
The invention provides a method for preparing vorapaxar intermediate, which belongs to the technical field of chemical drug synthesis. The method comprises the following steps: step 1: in a tetrahydrofuran solvent, a compound VRPX-100 is selectively reduced by borane dimethyl sulfide to generate alcohol VRPX-101; and step 2: a alcohol intermediate obtained in the step 1 is oxidized by sulfur trioxide pyridine without separation to obtain VRPX-200. The method of the invention stably increases the total reaction yield from 20-60% to 70-80%; reduces the difficulty of production operation and safety risk, and is suitable for industrial production of kilogram scale.
Owner:BEIJING XINLINGXIAN MEDICAL TECH DEV CO LTD
A method for preparing potassium tetrahydrofuran-3-trifluoroborate
InactiveCN104610332BCheap and easy to getThe reaction steps are simpleGroup 3/13 element organic compoundsBorane dimethylsulfideRoom temperature
The invention discloses a new method for preparing tetrahydrofuran-3-potassium trifluoroborate. The new method specifically comprises the following steps: preparing di-alpha-pinoborane by using dextro-alpha-pinene and a borane-dimethyl sulfide complex as raw materials, then reacting di-alpha-pinoborane with 2,5-dihydrofuran at room temperature, then using anhydrous acetaldehyde for reduction to generate dimethyl borate, without separating an intermediate, and subsequently reacting dimethyl borate with a saturated aqueous solution of KHF2 to generate the target product tetrahydrofuran-3-potassium trifluoroborate. The method has the obvious advantages that the reaction raw materials are easy to obtain; the reaction operation is simple; large-scale production is easy to achieve; the yield is high; the purity is good; the production cost is low.
Owner:成都安斯利生物医药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Preparation method of chiral S/R-3-ethoxy-4-methoxy-alpha[(methylsulfonyl)methyl] benzyl alcohol Preparation method of chiral S/R-3-ethoxy-4-methoxy-alpha[(methylsulfonyl)methyl] benzyl alcohol](https://images-eureka.patsnap.com/patent_img/97e6e5f8-76a4-40f2-bf53-c0138f8549ad/HDA0000860577330000011.PNG)
![Preparation method of chiral S/R-3-ethoxy-4-methoxy-alpha[(methylsulfonyl)methyl] benzyl alcohol Preparation method of chiral S/R-3-ethoxy-4-methoxy-alpha[(methylsulfonyl)methyl] benzyl alcohol](https://images-eureka.patsnap.com/patent_img/97e6e5f8-76a4-40f2-bf53-c0138f8549ad/HDA0000860577330000012.PNG)
![Preparation method of chiral S/R-3-ethoxy-4-methoxy-alpha[(methylsulfonyl)methyl] benzyl alcohol Preparation method of chiral S/R-3-ethoxy-4-methoxy-alpha[(methylsulfonyl)methyl] benzyl alcohol](https://images-eureka.patsnap.com/patent_img/97e6e5f8-76a4-40f2-bf53-c0138f8549ad/HDA0000860577330000021.PNG)
![Synthesis method of tert-butyl-8-oxa-3, 11-diazaspiro [5.6] dodecane-3-formate Synthesis method of tert-butyl-8-oxa-3, 11-diazaspiro [5.6] dodecane-3-formate](https://images-eureka.patsnap.com/patent_img/5ba9af85-40cf-40f8-94e7-4ff1101bb4c1/243538DEST_PATH_IMAGE004.png)
![Synthesis method of tert-butyl-8-oxa-3, 11-diazaspiro [5.6] dodecane-3-formate Synthesis method of tert-butyl-8-oxa-3, 11-diazaspiro [5.6] dodecane-3-formate](https://images-eureka.patsnap.com/patent_img/5ba9af85-40cf-40f8-94e7-4ff1101bb4c1/700298DEST_PATH_IMAGE002.png)
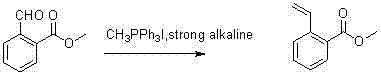
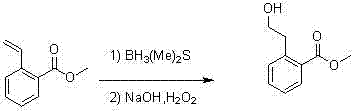

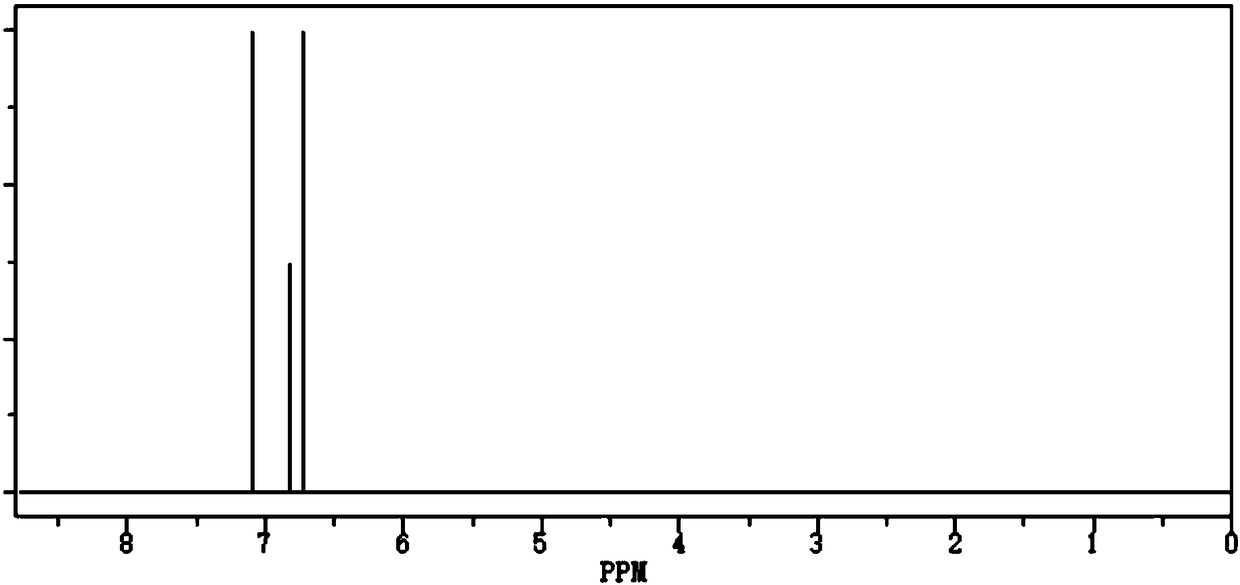

![The preparation method of chiral S or r-3-ethoxy-4-methoxy-α-[(methylsulfonyl) methyl] benzyl alcohol The preparation method of chiral S or r-3-ethoxy-4-methoxy-α-[(methylsulfonyl) methyl] benzyl alcohol](https://images-eureka.patsnap.com/patent_img/0e102024-f4f6-4a16-b6f8-e2154e2e9e14/HDA0000860577330000011.png)
![The preparation method of chiral S or r-3-ethoxy-4-methoxy-α-[(methylsulfonyl) methyl] benzyl alcohol The preparation method of chiral S or r-3-ethoxy-4-methoxy-α-[(methylsulfonyl) methyl] benzyl alcohol](https://images-eureka.patsnap.com/patent_img/0e102024-f4f6-4a16-b6f8-e2154e2e9e14/HDA0000860577330000012.png)
![The preparation method of chiral S or r-3-ethoxy-4-methoxy-α-[(methylsulfonyl) methyl] benzyl alcohol The preparation method of chiral S or r-3-ethoxy-4-methoxy-α-[(methylsulfonyl) methyl] benzyl alcohol](https://images-eureka.patsnap.com/patent_img/0e102024-f4f6-4a16-b6f8-e2154e2e9e14/HDA0000860577330000021.png)
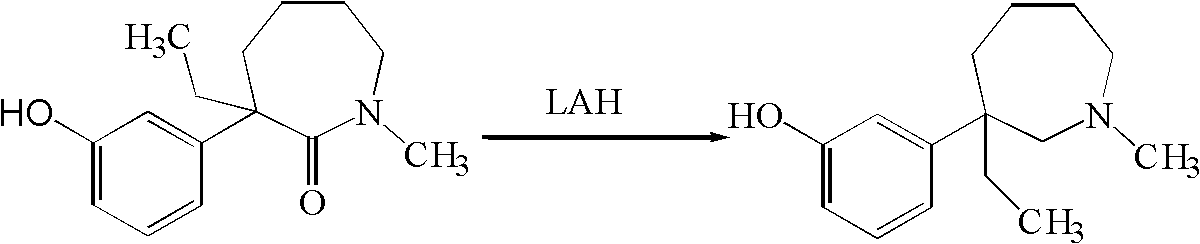
![Preparation method of 2,2,2-trifluoro-N-[(1S,4S)-4-hydroxy tetrahydronaphthalene-1-yl]-acetamide Preparation method of 2,2,2-trifluoro-N-[(1S,4S)-4-hydroxy tetrahydronaphthalene-1-yl]-acetamide](https://images-eureka.patsnap.com/patent_img/3a32558e-78b5-474f-99b0-b6491fa21ab4/DEST_PATH_IMAGE006.png)
![Preparation method of 2,2,2-trifluoro-N-[(1S,4S)-4-hydroxy tetrahydronaphthalene-1-yl]-acetamide Preparation method of 2,2,2-trifluoro-N-[(1S,4S)-4-hydroxy tetrahydronaphthalene-1-yl]-acetamide](https://images-eureka.patsnap.com/patent_img/3a32558e-78b5-474f-99b0-b6491fa21ab4/DEST_PATH_IMAGE006A.png)
![Synthesis method of (1R)-2-[[[2-(4-aminophenyl) ethyl] amino] methyl] benzyl alcohol Synthesis method of (1R)-2-[[[2-(4-aminophenyl) ethyl] amino] methyl] benzyl alcohol](https://images-eureka.patsnap.com/patent_img/4d95139d-e51d-4cdd-bf25-6316be0add90/RE-200312224637.png)
![Synthesis method of (1R)-2-[[[2-(4-aminophenyl) ethyl] amino] methyl] benzyl alcohol Synthesis method of (1R)-2-[[[2-(4-aminophenyl) ethyl] amino] methyl] benzyl alcohol](https://images-eureka.patsnap.com/patent_img/4d95139d-e51d-4cdd-bf25-6316be0add90/RE-DEST_PATH_IMAGE002.png)
![Synthesis method of (1R)-2-[[[2-(4-aminophenyl) ethyl] amino] methyl] benzyl alcohol Synthesis method of (1R)-2-[[[2-(4-aminophenyl) ethyl] amino] methyl] benzyl alcohol](https://images-eureka.patsnap.com/patent_img/4d95139d-e51d-4cdd-bf25-6316be0add90/RE-18898DEST_PATH_IMAGE003.png)